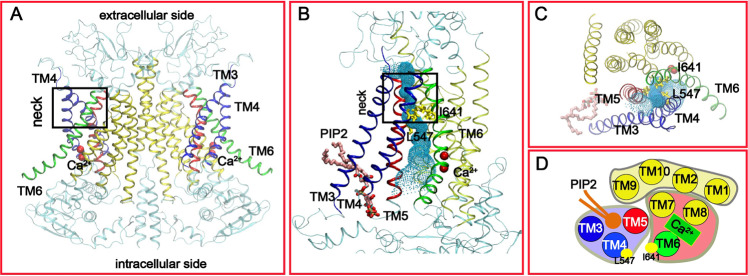
Fig. 1. Structural features of the Ca2+-bound mTMEM16A channel.
A The overall dimer structure with key TM helices highlighted (PDB: 5oyb). B, C Front and top views of the putative conducting pore. The pore profile calculated using HOLE39 is illustrated using the green dots. The two key hydrophobic residues, L547 and I641, in the “neck” region are shown in yellow sticks. The pore-lining TMs are colored in blue (3 and 4), red (5) and green (6), respectively. The other TMs are colored in yellow. The bound PIP2 molecule is represented as sticks in panels B and C. D Cartoon representation of the functional domain organization of TMs from the top. The pore-forming domain consists of the PIP2-binding regulatory module (TMs 3-5) and Ca2+-binding activation module (TMs 6–8). TMs 1, 2, 9, and 10 form the dimerization and supporting domain.