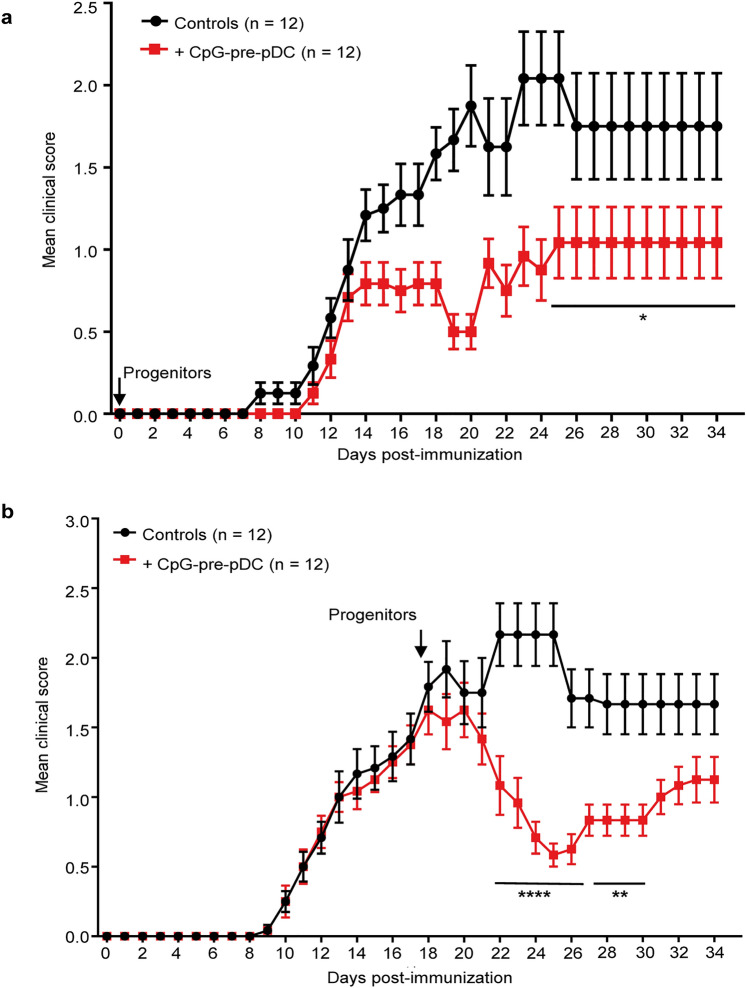
Figure 7.
Extended therapeutic window of CpG-pre-pDCs against EAE. CpG-pre-pDCs, 80,000 per recipient, were adoptively transferred either (a) at d-0 (n = 12 mice per group), or (b) at d-17 (n = 12 mice per group) after immunization. Clinical scores were assessed until day-35. Data are from 2 cumulated experiments. Statistical analysis was performed using two-way ANOVA with Bonferroni post-test, (a) d25–d34, *, p = 0.0453, (b) d22–d26, ****, p < 0.0001, d27–d30, **, p = 0.0021.