Fig. 2.
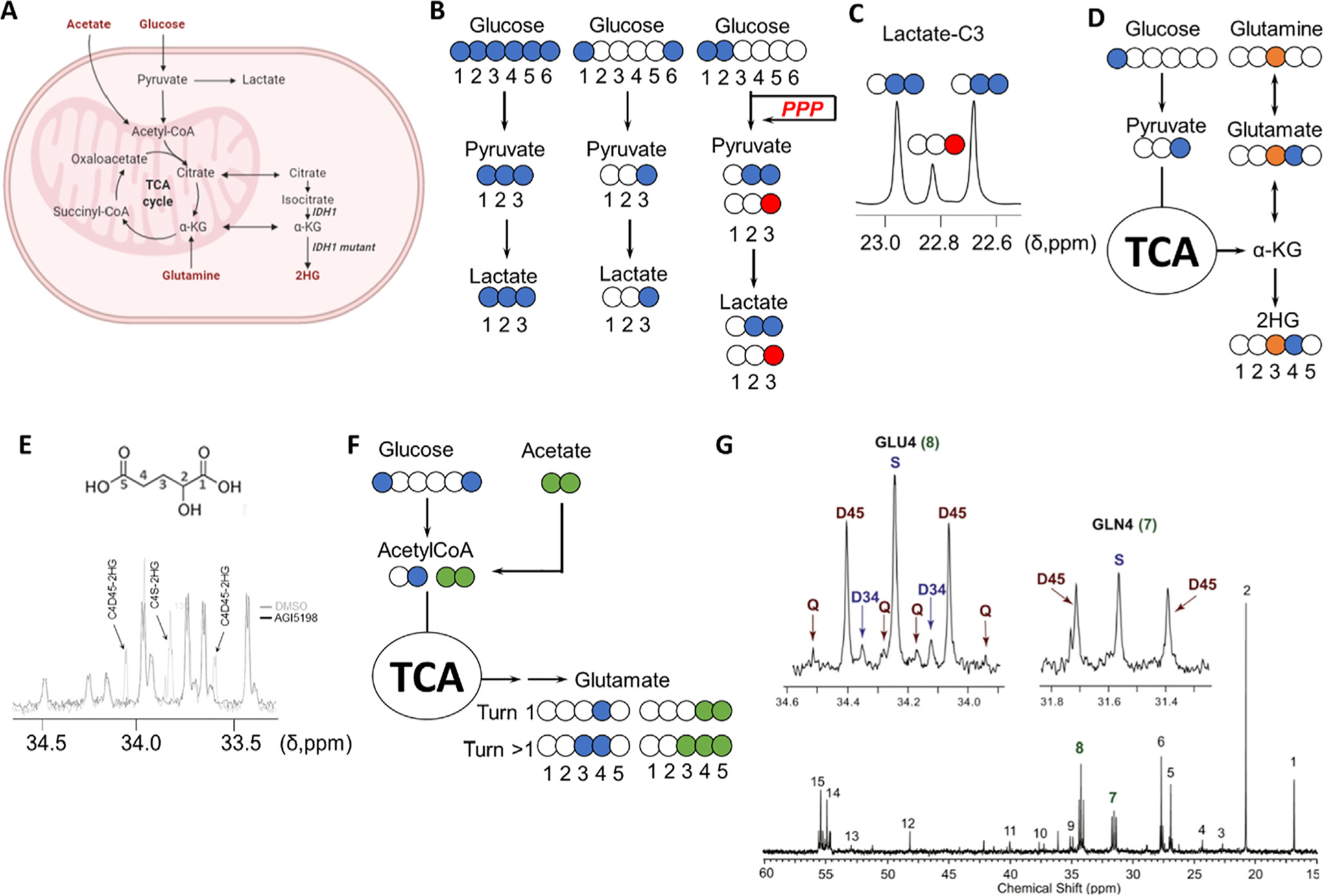
Investigation of metabolic rewiring in CNS malignancies. A, Key metabolic pathways involved in tumor metabolism. B, 13C tracing-based approaches to investigate the upregulation of glycolysis in cancer: blue colored circles denote the location of the 13C atoms in the substrates as well as in the subsequent metabolic products through glycolysis (blue) or pentose phosphate pathway (red). [1,2-13C]-glucose generates [2,3-13C]-lactate through glycolysis and [3-13C]-lactate if glucose-6P is diverted to the pentose phosphate pathway (PPP) by glucose-6P dehydrogenase, and once transformed into glyceraldehyde-3P, enters back into the glycolytic route. The contribution of each of those pathways to lactate formation can be quantified by comparing the areas under the resonance signals of lactate in a 13C spectrum, as the multiplet generated contains the information about the levels of both isotopologues. D, Analysis of the contribution of glucose and glutamine to 2HG synthesis in IDH1 mutant gliomas by examination of the resonances of C3 and C4 of 2HG from C3-13C-glutamine (orange circle) and C1-13C-glucose. E, Multiplet arising from 13C labeling of 2HG from TS603 cell line seeded in media containing [U-13C]-Glutamine and analyzed as described in [86]. 2HG structure with assignments displayed on top of the 13C spectrum [87]. F, Investigation of acetate contribution to the TCA through coadministration of [1,6-13C]-glucose (blue circles) and [1,2-13C]-acetate (green circles). (G) The multiplets attributable to incorporation of 13C into positions 3,4 and 5 (top) in both glutamine and glutamate C4 group serve to assign the specific contribution of each substrate (glucose and acetate) to the TCA. A 13C NMR GBM tumor spectrum after co-infusion is displayed. Chemical shift assignments can be found in the original article. Reproduced from [88] with permission.
