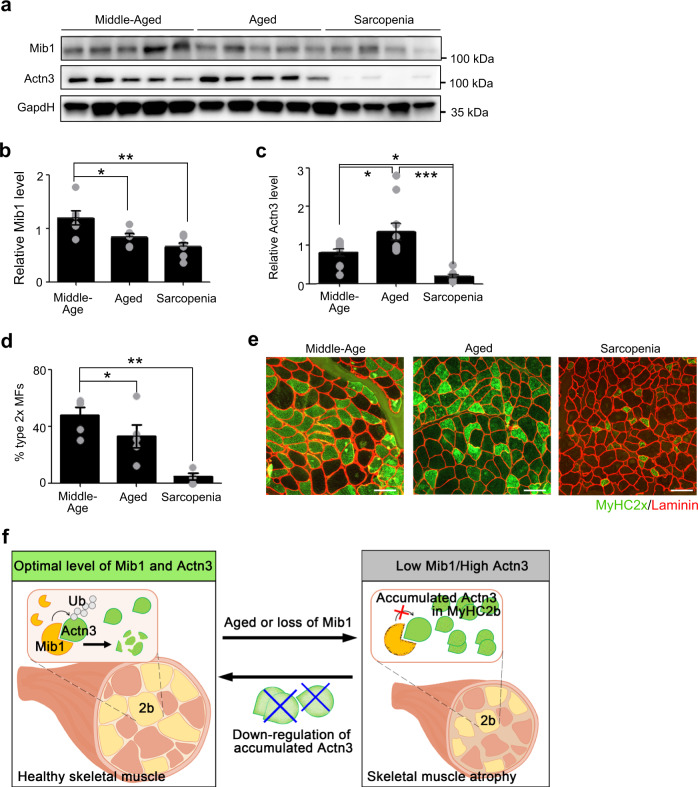
Fig. 6. Disturbed Mib1–Actn3 axis in human skeletal muscle with age.
a–c IB analysis (a) of Mib1 and Actn3 in vastus lateralis muscles of middle-aged (<60 years), aged (>60 years), and sarcopenia (>60 years and meet AGWS criteria), and quantification of Mib1 (b) and Actn3 (c). Please see Supplementary Fig. 7a for flow chart of selection of human subjects. The intensity of Mib1 and Actn3 expression at indicated group was quantified by densitometry. d, e Quantification of the percentage of type 2× glycolytic myofibers (d) and e representative images of IHC staining for MyHC2x (green; type 2× glycolytic myofibers) and laminin (red). Scale bars, 100 μm (e). Data are presented as means ± s.e.m. Data are shown as representatives of at least three independent experiments. n = 6 for middle-aged and aged group, and 8 for sarcopenic group (a–c). n = 5 for the middle-aged and aged group, and 4 for sarcopenic group (d). One-way ANOVA for (b–d). *P < 0.05; **p < 0.01; ***p < 0.001. f A proposed model. In healthy myofibers, Mib1 regulates Actn3, a Z-disk protein highly expressed in type 2 glycolytic myofibers, in a proteasome-dependent manner to maintain the optimal levels of Actn3. However, the age-associated changes in or loss of Mib1 in myofibers lead to the accumulation of Actn3 accompanied by the alteration of type 2 glycolytic myofibers, muscle atrophy, impaired muscle function, and, consequently, an acceleration of age-associated muscle atrophy. When the excessive accumulation of Actn3 is alleviated by the downregulation of Actn3 in myofibers, the muscle atrophy is ameliorated, suggesting that Actn3 can be a promising therapeutic target of age-associated muscle atrophy.