Abstract
Background
Sleep disturbances may increase risks of Alzheimer's disease (AD) and other dementias. Benign prostatic hyperplasia (BPH) is usually associated with lower urinary tract symptoms, including nocturia, and thereby disturbed sleep. We examined if men with BPH are at increased risk of AD and all-cause dementia.
Methods
In a Danish nationwide cohort (1996–2016), we identified 297,026 men with BPH, defined by inpatient or outpatient hospital diagnosis or by BPH-related surgical or medical treatment, and 1,107,176 men from the general population matched by birth year. We computed rates, cumulative incidences, and adjusted hazard ratios (HRs) of AD and all-cause dementia. Follow-up started 1 year after BPH diagnosis date/index date.
Findings
Median follow-up was 6·9 years (Interquartile range (IQR), 3·6 – 11·6 years] in the BPH cohort and 6·4 years (IQR: 3·4 – 10·8 years) in the comparison cohort. The cumulative 1–10 year risk of AD was 1·15% [95% confidence interval (CI), 1·11–1·20], in the BPH cohort and 1·00% (95% CI, 0·98 – 1·02) in the comparison cohort. The adjusted 1–10–year hazard ratios were 1·16 (95% CI: 1·10–1·21) for AD and 1·21 (95% CI: 1·17–1·25) for all-cause dementia. From >10 years up to 21 years of follow-up, BPH remained associated with 10%- 20% increased risk of AD and all-cause dementia.
Interpretation
During up to 21 years of follow-up, men with BPH had persistently higher risk of AD and all-cause dementia compared with men in the general population. Our results identify BPH as a common, potentially remediable disorder associated with dementia risk.
Funding
Lundbeckfonden, Aarhus University Research Foundation, and the National Institutes of Health.
Keywords: Benign prostatic hyperplasia, Alzheimer's disease, Dementia, Lower urinary tract symptoms, Nationwide cohort study
Research in context.
Evidence before this study
The pathological cascade of Alzheimer's disease may begin with the accumulation of amyloid-β and hyperphosphorylated tau which is normally cleared from the brain during sleep. Sleep problems may thus contribute to the development of Alzheimer's disease. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia include nocturia and thereby disrupted sleep. Using the terms “benign prostatic hyperplasia”, “dementia”, and “Alzheimer's disease”, we searched PubMed and found no study that had considered whether benign prostatic hyperplasia is associated with Alzheimer's disease or all-cause dementia.
Added value of this study
In this nationwide population-based cohort study, we found a 16% increased 10–year risk of Alzheimer's disease and a 21% increased risk of all-cause dementia in men with benign prostatic hyperplasia. The increased risks persisted across various sensitivity analyses and remained elevated during more than 20 years of follow-up. These findings support the hypothesis that disrupted sleep may contribute to risk of Alzheimer's disease and other dementias.
Implications of all the available evidence
Since benign prostatic hyperplasia and Alzheimer's disease are two common diseases, an association could have major public health impact. This study thus points to a need for attention to sleep patterns in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia and to consider interventions that decreases nocturia and sleep fragmentation.
Alt-text: Unlabelled box
1. Introduction
Alzheimer's disease (AD), the most common cause of dementia, affects approximately 44 million older adults worldwide [1]. An early step in AD pathogenesis is accumulation of amyloid-β and hyperphosphorylated tau proteins within vulnerable brain regions [2]. The glymphatic system is a major pathway for removal of these and other toxic substances. Glymphatic flow increases during sleep, and sleep deprivation and sleep fragmentation are associated with increased AD risk [3].
Sleep disturbance is a common symptom and is related to various factors, including the use of caffeine and alcohol, sleep habits, and comorbid diseases. Nocturia leads to sleep deprivation and sleep fragmentation and is among the most common causes of secondary insomnia [4]. Approximately 70% of men with symptomatic benign prostatic hyperplasia (BPH) experience nocturia [5]. If disturbed sleep is indeed a risk factor for AD, BPH may lead to increased occurrence of AD in men by interrupting sleep and thereby reducing amyloid-β and hyperphosphorylated tau efflux from the brain. As lower urinary tract symptoms suggestive of BPH are a common condition [6], any increased risk of AD could have major public health implications.
We used Danish population-based medical registries to examine the hypothesis that BPH is associated with AD risk. At the same time, we also examined associations between BPH and dementia of any cause.
2. Materials and methods
2.1. Data sources and study population
We conducted a nationwide cohort study within the Danish population of approximately 5·7 million people. All residents are provided free tax-supported access to health care. Since 1968 a unique personal identifier, the Civil Registration Number, has been assigned to all residents at birth or upon immigration by the Civil Registration System [7]. This identifier allows unambiguous data linkage at the individual level. The Civil Registration System also tracks changes in vital status and migration for the entire population.
The Danish National Patient Registry (DNPR) has recorded all inpatient admissions to all Danish hospitals since 1977 and hospital outpatient and emergency room visits since 1995 [8]. The Danish Psychiatric Central Research Registry has recorded all hospitalizations in psychiatric departments since 1970 and all psychiatry-related hospital outpatient or emergency room visits since 1994 [9].
The Danish National Prescription Database contains complete data on prescription medications dispensed from community pharmacies and hospital-based outpatient pharmacies in Denmark since 1995 [10].
2.2. BPH and comparison cohorts
We identified all men with a hospital inpatient or outpatient clinic diagnosis of BPH, and/or a transurethral resection of the prostate (TURP) and/or at least one prescription for BPH-related medical treatments, defined as alpha-receptor blockers or 5-alpha reductase inhibitors from 1996 to 2016 (see Appendix for codes). They were included in the cohort at the date of the first of these criteria. Thus, the cohort members who were identified based on a BPH diagnosis code could later receive medication and/or TURP as well and vice versa. To identify only incident BPH and avoid left truncation of the data, we excluded men who also had BPH recorded during 1977–1995 and men with BPH-related prescriptions in 1995–1996. As diagnosis codes in DNPR have been coded according to ICD-10 since 1994, [9] we identified men using a diagnosis of BPH without direct knowledge of the presence or extent of lower urinary tract symptoms. We, therefore, have used the term BPH throughout the manuscript, while acknowledging that this term may largely have been replaced in the literature by lower urinary tract symptoms suggestive of BPH.
We excluded men with dementia, mild cognitive impairment, or amnestic syndrome before BPH diagnosis date. The BPH diagnosis date was the first inpatient or outpatient BPH diagnosis date, the TURP date, or the first BPH-related prescription date, whichever came first.
The comparison cohort consisted of up to 4 birth-year-matched men from the general population for each BPH patient. Comparison men were not allowed to have a diagnosis of BPH, a TURP, a filled prescription for BPH-related treatment, or a diagnosis of dementia, mild cognitive impairment, or amnestic syndrome recorded before the BPH diagnosis date of the matched patient (index date).
2.3. Outcome
The main study outcome was a first-time inpatient or outpatient clinic diagnosis of AD. Secondarily, we examined risk of all-cause dementia (see Appendix for codes).
2.4. Baseline variables and potential confounders
The Civil Registration System provided information on birth year and emigration data. The DNPR provided information on previous cardiovascular diseases (myocardial infarction, heart failure, peripheral vascular disease, atrial fibrillation/flutter, and stroke), diabetes mellitus, hyperlipidemia, hypertension, renal failure and prostate cancer. As a proxy measure for smoking we retrieved information on chronic obstructive pulmonary disease (COPD) diagnoses.
Both nonsteroidal anti-inflammatory drugs (NSAIDs) [11] and higher serum testosterone [12] are associated with increased risk of BPH and reduced risk of AD [13,14]. We retrieved prescriptions for NSAIDs (at least one prescription filled within 6 months before index date) and testosterone (at least one prescription or a registered treatment code in DNPR any time before index date). We also retrieved information on prescriptions for antihypertensive drugs (at least one prescription filled within 6 months before index date).
From the Integrated Database for Labour Market Research, we retrieved highest attained education, personal gross income, and employment status during the year preceding the index date, and highest level of achieved education [15]. Missing data were included as separate categories.
2.5. Statistical analysis
Follow-up began one year after the index date, to avoid dementia diagnoses detected as part of diagnostic work-up for BPH, and continued until the first diagnosis of dementia, death, emigration, or study end (December 31, 2016), whichever occurred first. Comparisons who developed BPH during follow-up were censored and transferred into the BPH cohort one year after BPH diagnosis. We computed incidence rates per 1000 person-years and unadjusted and adjusted hazard ratios (HRs) of AD and all-cause dementia. We used stratified Cox regression analysis to control for age and calendar period and we adjusted for education, personal gross income, and employment status as indicators of cognitive reserve [16]. The metabolic syndrome is associated with both BPH and AD [17,18] In addition, lower urinary tract symptoms may be associated with increased risk of cardiovascular events [19], which may contribute to early cognitive decline and dementia [20]. Therefore, we adjusted for cardiovascular disease, diabetes mellitus, hyperlipidemia, hypertension, and COPD before the date of BPH diagnosis. We also adjusted for use of antihypertensive drugs, use of NSAIDs, and testosterone, respectively.
We included renal failure, which is associated with both BPH [21] and dementia risk, [22] as a time-varying exposure using Cox regression analysis. Although TURP may not improve sleep disruption in men with BPH [5] we explored TURP as a time-varying exposure in the same manner.
We computed and plotted the 1–10- and >10–21-year cumulative incidence of dementia, considering death as a competing risk. We stratified analyses by presence of the included chronic diseases. In these analyses, we dissolved the matching in order to directly compare the BPH patients with a specific chronic disease with comparisons with the same chronic disease. We adjusted for age and calendar year (as continuous variables) and used robust variance estimates when computing confidence intervals (CIs).
For the Cox models, we assessed and accepted the proportional hazards assumption graphically using log-log plots.
2.6. Sensitivity analyses
We conducted several sensitivity analyses to ensure robustness of our results. We assessed possible period effects by stratifying analyses for the calendar periods 1996–2002, 2003–2009, and 2010–2016 while limiting follow-up to 10 years. We examined whether dementia risk differed between BPH patients identified based on a hospital-related diagnosis and those identified based on prescriptions and whether restriction to men without chronic comorbidity affected the estimates. We also examined whether dementia risk differed by type of medical treatment for BPH and whether it increased with increasing intensity of medical treatment. In this latter analysis we categorized treatment intensity measured as prescriptions per year into quartiles. In another sensitivity analysis, men from the general population remained in the comparison cohort if they developed BPH. Finally, we excluded BPH patients and comparisons with either prostate cancer or renal failure at baseline and censored those who developed prostate cancer or renal failure during follow-up.
All statistical analyses were conducted using the SAS statistical software package, v. 9.4 (SAS Institute, Cary, NC). The study was approved by the Danish Data Protection Agency (record number: 2014‐54‐0922/KEA‐1-15). The study adhered to the RECORD guidelines.
2.7. Role of the funding source
None. The researchers acted independently from the study sponsors in all aspects of this study. MN, HTS, and EHP had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
3. Results
Of 331 844 men with BPH, we included 297 026 in our cohort one year after BPH diagnosis. Of these, we identified 68 601 (23·1%) based on a BPH diagnosis, 3823 (1·3%) on a TURP procedure, and 224 602 (75·6%) on a BPH-related prescription.
Median age at start of follow-up was 67·5 years [interquartile range (IQR) 59·2 – 75·2 years] (Table 1). The comparison cohort included 1107,176 birth-year-matched men without BPH. Slightly more men with BPH received a state pension, compared with men in the general population cohort, while income and education were similarly distributed between the two groups. Men with BPH had a higher prevalence of the included chronic diseases than comparison men (Table 1).
Table 1.
Baseline characteristics of men with a first benign prostatic hyperplasia (BPH) diagnosis, defined as either a hospital-based diagnosis, transurethral resection of the prostate, or a BPH related prescription in Denmark in 1996–2016 and men in the comparison cohort who were alive and started follow-up, one year after the BPH diagnosis/index date.
| Men with BPH n = 297 026 N (%) | Comparison cohort n = 1 107 176 N (%) | |
|---|---|---|
| Age groups, years | ||
| <60 | 76 434 (25·7) | 301 002 (27·2) |
| 60–69 | 92 960 (31·3) | 356 549 (32·2) |
| 70–79 | 86 583 (29·1) | 315 891 (28·5) |
| 80+ | 41 049 (13·8) | 133 734 (12·1) |
| Education | ||
| Primary school (≤9 years) | 93 730 (31·6) | 363 633 (32·8) |
| Secondary/vocational (10–12 years) | 110 921 (37·3) | 423 134 (38·2) |
| Higher education (>12 years) | 57 074 (19·2) | 201 718 (18·2) |
| Missing | 35 301 (11·9) | 118,691 (10·7) |
| Employment | ||
| Employed/self-employed | 110 188 (37·1) | 446 889 (40·4) |
| Unemployed | 9653 (3·2) | 35 693 (3·2) |
| Early retirement pensioner | 39 941 (13·4) | 135 691(12·3) |
| Retired | 136 012 (45·8) | 480 223 (43·4) |
| Missing | 1232 (0·4) | 8680 (0·8) |
| Income | ||
| Low (1st quartile) | 71 195 (24·0) | 260 619 (23·5) |
| Medium-low (2nd quartile) | 72 181 (24·3) | 265 316 (24·0) |
| Medium-high (3rd quartile) | 74 477 (25·1) | 279 183 (25·2) |
| High (4th quartile) | 78 631 (26·5) | 298 373 (26·9) |
| Missing | 542 (0·2) | 3685 (0·3) |
| Calendar period of BPH diagnosis/index date | ||
| 1996–2002 | 109 844 (37·0) | 402 952 (36·4) |
| 2003–2009 | 97 017 (32·7) | 362 960 (32·8) |
| 2010–2016 | 90 165 (30·4) | 341 264 (30·8) |
| Comorbidities at baseline | ||
| Cardiovascular disease | 63 431 (21·4) | 195 700 (17·7) |
| Stroke | 17 069 (5·7) | 47 579 (4·3) |
| Diabetes mellitus | 19 964 (6·7) | 54 720 (4·9) |
| Hyperlipidemia / hypercholesterolemia | 17 009 (5·7) | 49 377 (4·5) |
| Hypertension | 43 511 (14·6) | 111 223 (10·0) |
| Obesity | 7085 (2·4) | 17 488 (1·6) |
| Chronic obstructive pulmonary disease | 21 809 (7·3) | 60 033 (5·4) |
| Renal failure | 4188 (1·4) | 6436 (0·6) |
| Prostate cancer | 6675 (2·2) | 11 539 (1·0) |
| Use of medication at or before baseline | ||
| NSAIDs* | 54 648 (18·4) | 139 718 (12·6) |
| Testosterone | 1699 (0·6) | 4052 (0·4) |
| Follow-up, years | ||
| Median, interquartile range | 6·9 (3·6–11·6) | 6·4 (3·4–10·8) |
| Events during follow up | ||
| Transurethral resection of the prostate** | 49 418 (16·6) | – |
| Renal failure | 16 854 (5·7) | 32 952 (3·0) |
| Prostate cancer | 29 453 (9·9) | 31 506 (2·8) |
*within 6 months before index date **within 5 years before or after index date.
We followed both cohorts, for a median of 6·9 years (IQR, 3·6 – 11·6 years) and a median of 6·4 years (IQR: 3·4 – 10·8 years), respectively. During follow-up, 5·7% of men in the BPH cohort developed renal failure and 9·9% developed prostate cancer compared with 3·0% and 2·8%, respectively among comparisons.
During follow-up, 7907 men with BPH and 20 745 members of the comparison cohort were diagnosed with incident dementia of any cause. Of these, 3603 men with BPH and 9946 comparisons had AD. The 1–10 year rates for AD (per 1000 person-years) were 1·55 (95% CI, 1·49–1·61) in the BPH cohort and 1·25 (95% CI, 1·23–1·28) in the comparison cohort, corresponding to a 1–10 year adjusted HR of 1·16 (95% CI, 1·10–1·21) for AD. For >10–21-years of follow up the adjusted HR was 1·10 (95% CI, 0·99–1·21).
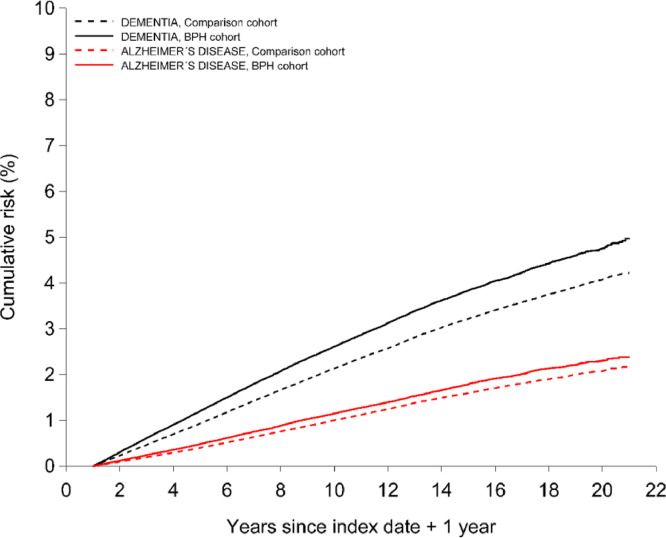
The cumulative risk of AD was consistently higher in the BPH cohort than in comparisons (Fig. 1). During 1 to 10 years of follow-up, the cumulative risk of AD was 1·15% (95% CI, 1·11–1·20) in the BPH cohort and 1·00% (95% CI, 0·98 –1·02) in the comparison cohort, corresponding to a 1–10 year risk difference of 0·15%. During >10 to 21 years of follow-up, the cumulative risk of AD increased to 2·00% (95% CI, 1·84%−2·18%) in the BPH cohort and to 1·69% (95% CI, 1·58%−1·80%) in the comparison cohort and a risk difference of 0·31%.
Fig. 1.
Cumulative incidence of Alzheimer's disease and all-cause dementia among men with benign prostatic hyperplasia and matched comparisons.
BPH was similarly associated with increased risk of all cause dementia with 1–10 year adjusted HR of 1·21 (95% CI, 1·17–1·25) and >10–21 year HR of 1·18 (95% CI, 1·10–1·27) (Table 2).
Table 2.
Numbers, rates, and hazard ratios of Alzheimer's disease and all-cause dementia in different periods of follow-up in men with benign prostatic hyperplasia (BPH) compared with a matched cohort of men without BPH.
| BPH | Comparison cohort | Unadjusted* HR(95% CI) | Adjusted*† HR(95% CI) |
|||
|---|---|---|---|---|---|---|
| No. of events | Rate per 1000 PY(95% CI) | No. of events | Rate per 1000 PY(95% CI) | |||
| Alzheimer's disease | ||||||
| 1–10 years | 2572 | 1·55 (1·49–1·61) | 7390 | 1·25 (1·23–1·28) | 1·13 (1·08–1·19) | 1·16 (1·10–1·21) |
| >10–21 years | 1031 | 2·44 (2·29–2·59) | 2556 | 1·96 (1·88–2·04) | 1·08 (0·98–1·19) | 1·10 (0·99–1·21) |
| All-cause dementia | ||||||
| 1–10 years | 5943 | 3.59 (3.50–3.68) | 16 124 | 2.74 (2.69–2.78) | 1.21 (1.17–1.25) | 1.21 (1.17–1.25) |
| >10–21 years | 1964 | 4.65 (4.44–4.86) | 4621 | 3.54 (3.44–3.65) | 1.18 (1.10–1.27) | 1.18 (1.10–1.27) |
* Corrected for age, time period of diagnosis/index date by study design.
†adjusted for cardiovascular disease, diabetes mellitus, hyperlipidemia, hypertension, obesity, chronic obstructive pulmonary disease, use of antihypertensive drugs, use of NSAIDs, income in the year before index date, and employment status in the year before index date, and renal failure (time-varying). Except for renal failure all included covariates were measured at the date of BPH diagnosis/index date.
Tables 3 and 4 present the associations between BPH and AD and all-cause dementia, stratified by presence of selected pre-existing chronic diseases. In men with pre-existing cardiovascular disease or diabetes, BPH remained associated with increased risk of AD (Table 3) and all-cause dementia (Table 4). In men with hyperlipidemia or hypertension this association, however, was no longer evident. Including renal failure as a time-varying covariate in the stratified analyses did not substantially change the estimates (data not shown).
Table 3.
Cumulative 1–10 and >10–21 year risks and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) of Alzheimer's disease in patients with benign prostatic hyperplasia (BPH) compared with men without BPH stratified by history of selected chronic diseases and by length of follow-up.
| 1–10 -year cumulative risk | >10–21 -year cumulative risk | |||||
|---|---|---|---|---|---|---|
| BPH cohort % (95% CI) |
Comparison cohort% (95% CI) | Adjusted* HR (95% CI) | BPH cohort% (95% CI) | Comparison cohort% (95% CI) | Adjusted* HR (95% CI) | |
| History of cardiovascular disease | ||||||
| Yes | 1·30 (1·20–1·41) | 1·19 (1·13–1·25) | 1·16 (1·06–1·28) | 2·08 (1·65–2·60) | 1·70 (1·48–1·94) | 1·10 (0·90–1·36) |
| No | 1·11 (1·06–1·16) | 0·96 (0·94–0·99) | 1·11 (1·06–1·17) | 2·00 (1·82–2·19) | 1·69 (1·57–1·81) | 1·11 (1·02–1·20) |
| History of diabetes | ||||||
| Yes | 1·00 (0·84–1·18) | 0·99 (0·88–1·11) | 1·16 (0·95–1·41) | 3·39 (0·96–8·47) | 1·13 (0·80–1·54) | 1·53 (0·97–2·41) |
| No | 1·16 (1·11–1·21) | 1·00 (0·98–1·02) | 1·12 (1·07–1·17) | 1·98 (1·83–2·15) | 1·70 (1·59–1·81) | 1·10 (1·02–1·18) |
| History of hyperlipidemia | ||||||
| Yes | 1·11 (0·91–1·35) | 1·18 (1·04–1·33) | 0·97 (0·78–1·20) | 1·48 (0·81–2·52) | 2·09 (1·45–2·92) | 0·66 (0·37–1·16) |
| No | 1·15 (1·11–1·20) | 0·99 (0·97–1·02) | 1·13 (1·08–1·18) | 2·01 (1·85–2·19) | 1·68 (1·58–1·79) | 1·12 (1·04–1·20) |
| History of hypertension | ||||||
| Yes | 1·16 (1·03–1·29) | 1·23 (1·14–1·33) | 1·05 (0·92–1·19) | 1·23 (0·90–1·64) | 1·27 (0·99–1·60) | 0·99 (0·70–1·40) |
| No | 1·15 (1·10–1·20) | 0·98 (0·96–1·00) | 1·14 (1·08–1·19) | 2·06 (1·88–2·24) | 1·70 (1·59–1·81) | 1·11 (1·03–1·20) |
| History of chronic obstructive pulmonary disease | ||||||
| Yes | 0·89 (0·75–1·04) | 0·99 (0·89–1·10) | 0·98 (0·81–1·18) | 2·04 (1·37–2·92) | 2·29 (1·53–3·30) | 1·08 (0·73–1·59) |
| No | 1·17 (1·12–1·22) | 1·00 (0·98–1·02) | 1·13 (1·08–1·19) | 2·00 (1·83–2·18) | 1·68 (1·57–1·79) | 1·11 (1·03–1·19) |
* Adjusted for age, time period of diagnosis/index date, income and employment status in the year before index date, obesity, renal failure and the diseases presented in the table (except the stratifying variable). All included covariates were measured at the date of BPH diagnosis/index date.
Table 4.
Cumulative 1–10 and >10–21 year risks and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) of all-cause dementia in patients with benign prostatic hyperplasia (BPH) compared with men without BPH stratified by history of selected chronic diseases and by length of follow-up.
| 1–10 -year cumulative risk | >10–21 -year cumulative risk | |||||
|---|---|---|---|---|---|---|
| BPH cohort% (95% CI) | Comparison cohort% (95% CI) | Adjusted* HR (95% CI) | BPH cohort% (95% CI) | Comparison cohort% (95% CI) | Adjusted* HR (95% CI) | |
| History of cardiovascular disease | ||||||
| Yes | 3.40 (3.24–3.57) | 3.06 (2.96–3.16) | 1.17 (1.11–1.24) | 4.32 (3.65–5.07) | 3.51 (3.17–3.87) | 1.16 (1.01–1.33) |
| No | 2.40 (2.33–2.48) | 1.95 (1.91–1.98) | 1.19 (1.15–1.23) | 3.79 (3.48–4.11) | 2.97 (2.82–3.12) | 1.17 (1.10–1.23) |
| History of diabetes | ||||||
| Yes | 2.91 (2.64–3.21) | 2.70 (2.52–2.89) | 1.17 (1.04–1.32) | 5.03 (2.22–9.56) | 3.20 (2.57–3.94) | 1.15 (0.85–1.55) |
| No | 2.59 (2.53–2.66) | 2.11 (2.07–2.14) | 1.19 (1.15–1.22) | 3.83 (3.55–4.13) | 3.01 (2.87–3.16) | 1.17 (1.11–1.23) |
| History of hyperlipidemia | ||||||
| Yes | 2.31 (2.01–2.63) | 2.32 (2.13–2.53) | 1.02 (0.88–1.19) | 3.56 (2.40–5.07) | 4.50 (3.20–6.11) | 0.85 (0.59–1.24) |
| No | 2.63 (2.56–2.70) | 2.13 (2.09–2.16) | 1.19 (1.16–1.23) | 3.85 (3.57–4.16) | 3.00 (2.87–3.14) | 1.17 (1.11–1.24) |
| History of hypertension | ||||||
| Yes | 2.74 (2.55–2.93) | 2.75 (2.61–2.89) | 1.09 (1.00–1.18) | 2.84 (2.32–3.45) | 3.13 (2.48–3.88) | 1.08 (0.86–1.35) |
| No | 2.60 (2.53–2.67) | 2.08 (2.04–2.11) | 1.20 (1.16–1.24) | 3.91 (3.62–4.23) | 3.01 (2.87–3.16) | 1.17 (1.11–1.24) |
| History of chronic obstructive pulmonary disease | ||||||
| Yes | 2.24 (2.02–2.47) | 2.19 (2.04–2.34) | 1.10 (0.98–1.24) | 3.61 (2.74–4.64) | 3.31 (2.46–4.34) | 1.23 (0.92–1.64) |
| No | 2.64 (2.57–2.71) | 2.13 (2.10–2.17) | 1.19 (1.16–1.23) | 3.86 (3.57–4.16) | 3.01 (2.87–3.16) | 1.17 (1.10–1.23) |
*Adjusted for age, time period of diagnosis/index date, income and employment status in the year before index date, obesity, renal failure and the diseases presented in the table (except the stratifying variable). All included covariates were measured at the date of BPH diagnosis/index date.
Although TURP reduces lower urinary tract symptoms, it often does not effectively treat nocturia [5], possibly because nocturnal polyuria is also common in men with lower urinary tract symptoms [23]. Thus, as expected, undergoing TURP did not change the relative risk of dementia. For AD, the adjusted 1–10 year HRs were 1·15 (95% CI, 1·04–1·28) among men who underwent TURP and 1·15 (95% CI, 1·08–1·21) among those who did not. For all-cause dementia, the 1–10 year risks were 1·19 (95% CI, 1·11–1·27) in men with BPH who had undergone TURP compared with 1·21 (95% CI, 1·17–1·26) in men with BPH without TURP.
3.1. Sensitivity analyses
In the analysis stratified by calendar period of BPH diagnosis, the adjusted 1–10 year HRs of AD were 1·16 (95% CI, 1·07–1·24) for 1996–2002, 1·15 (95% CI, 1·06–1·24) for 2003–2009, and 1·12 (95% CI, 0·97–1·29) for 2010–2016. Risk of AD did not vary between those identified based on a BPH diagnosis and those identified based on a BPH-related prescription; 1–10 year HRs were 1·18 (95% CI, 1·07–1·31) and 1·14 (1·07–1·21), respectively. Stratified by type of BPH-related prescription, the cumulative risk of AD during 1 to 10 years of follow-up was 1·15 (95% CI: 1·09 - 1·21) for users of alpha-receptor blockers and 1·15 (95% CI: 1·01 - 1·30) in users of 5-alpha reductase inhibitors. For all-cause dementia, these estimates were 2·53 (95% CI: 2·45 - 2·61) and 2·78 (95% CI: 2·57 - 3·01), respectively.
Restricting to men with none of the included baseline comorbidities, did not change the estimates substantially; the 1–10–year adjusted HRs were 1·18 (95% CI, 1·10–1·26) for AD and 1·25 (95% CI, 1·19–1·32) for all-cause dementia. Keeping men who developed BPH during follow-up in the comparison cohort decreased the 1–10 year adjusted HR of AD only slightly from 1·15% (95% CI, 1·11–1·20) to 1·13 (95% CI, 1·07–1·18). Excluding and censoring men with prostate cancer or renal failure did not change the estimates substantially; the 1–10 year adjusted HR of AD was 1.19 (95% CI, 1.13–1.26).
4. Discussion
In this large population-based cohort study with more than 20 years of follow-up, men with BPH had about 15% higher risk of AD compared with men without BPH. The increased risk persisted throughout follow-up despite adjustment for pre-existing cardiovascular and other metabolic diseases, COPD, educational attainment, and socioeconomic factors. The increased AD risk was similar for men with inpatient and outpatient hospital-diagnoses of BPH and men identified on the basis of BPH prescription medications. These findings support our hypothesis that BPH might contribute to AD risk through nocturia, causing sleep fragmentation and sleep deprivation and thereby interfering with glymphatic flow. Men with BPH also had a 20% increased risk of all-cause dementia, and glymphatic flow may also be important in removing toxic substances implicated in other neurodegenerative disorders [24].
The similar relative risks of AD in men with BPH with and without TURP is consistent with previous findings that TURP does not effectively treat nocturia [5]. If nocturia persists due to nocturnal polyurea [23] then restricting the amount of fluid-intake before bedtime could potentially be a treatment option. Nocturnal polyuria cannot be treated with alpha-blockers, which is consistent with findings in a recent Korean cohort study among 59,263 men with BPH in which type and duration of alpha-blocker use was not associated with a risk of dementia [25].
BPH with lower urinary tract symptoms is associated with increased risk of cardiovascular diseases [26,27]. When we took pre-existing cardio-metabolic diagnoses and COPD into account, HRs for AD and all-cause dementia were only marginally affected, suggesting that these factors did not, to a large degree, explain the association between BPH and dementia. Still, residual confounding may have affected our relative risk estimates. We lacked information on smoking, an important risk factor for AD [28]. Smoking is a weak risk factor for BPH [29] and our analyses restricted to men with a diagnosis of COPD, as an indirect marker of smoking, continued to show an association between BPH and dementia. We also lacked information on antimuscarinic drugs which are used for overactive bladder symptoms and could be used more often among med with BPH than among men from the background population and which may also affect cognitive function. A recent meta-analysis found anticholinergic use for at least 3 months compared with no use yielded a rate ratio for incident dementia of 1.46 (95% CI: 1.17–1.81) [30]. If men with BPH are 2.5 times more likely to use antimuscarinic drugs compared with men from the general population, an estimate derived from unpublished data in Denmark, a rate ratio for incident dementia of 1.4 would account for our observed association. We therefore cannot rule out that the observed observation between BPH and dementia is at least partly explained by use of antimuscarinic drugs. On the other hand, patients with overactive bladder may also have nocturia and disturbed sleep, a finding that might partly explain reported increased risk of AD and dementia in patients receiving antimuscarinic drugs.
We observed a higher incidence of prostate cancer in men with BPH than in the comparisons. Cancer diagnoses have, however, been related to a slightly lower risk of a dementia diagnosis [31] and censoring men who had a prostate cancer diagnosis did not change our estimates.
Use of nationwide medical registries allowed us to conduct a large cohort study with long and virtually complete follow-up. Still, our study has some weaknesses that should be considered. Our results do not address associations between disrupted sleep in women and Alzheimer risk. We used diagnosis codes recorded in an inpatient or outpatient hospital-based setting to identify BPH and these codes may not be entirely accurate [8]. However, the positive predictive values of other diagnostic codes in the group of urogenital diseases are between 75% and 100% in DNPR [8]. Moreover, our results based on BPH prescriptions—which helped identify men with lower urinary tract symptoms not diagnosed in the hospital-based setting— were similar to those based on BPH diagnoses.
We lacked information on severity of lower urinary tract symptoms in the BPH patients, and we were therefore unable to examine whether more severe nocturia was associated with higher dementia risk, a finding that could have strengthened our conclusion. Although we assume that most BPH patients diagnosed in an inpatient or outpatient hospital setting were likely to have relatively severe symptoms, we may also have included some men with BPH but without nocturia. If sleep disturbances are a cause of AD, including men with BPH without nocturia would lead to a lower estimate of AD risk and would thereby attenuate our relative estimates. Also, a European survey based on 455 general practitioners in Europe who were asked to provide data on the last two BPH patients who consulted their practice found that among 886 men with BPH, 71% reported nocturia at time of diagnosis [32]. We therefore expect that a high proportion of our study population have nocturia. BPH diagnoses by general practitioners are not captured in the DNPR, and we likely missed men with BPH diagnosed by their general practitionerand did not receive treatment. These men with presumably milder lower urinary tract symptoms could potentially have been included in the comparison population. Such misclassifications would reduce the magnitude of the association between BPH and AD and can therefore not explain our findings. Some men without BPH could have had nocturia from other causes, such as nocturnal polyuria [23]. However, resulting sleep disruption in the comparison cohort would have also attenuated the observed association between BPH and AD.
Men with BPH may have closer contact to the hospital system than men without BPH, increasing the likelihood of receiving a dementia diagnosis. To minimize such detection bias, we excluded dementia diagnoses during the first year of follow-up. We further stratified follow-up by time periods, assuming that detection bias would decrease over time. However, risks remained elevated even after 10 years of follow-up. Moreover, we stratified our study population by presence of other chronic diseases. We expect that men with, for example, diabetes will be in closer contact with the health care system regardless of a BPH diagnosis and our finding of an association between BPH and dementia also among men with diabetes to some extent speaks against surveillance bias as the major explanation for our findings.
In conclusion, the higher risk of AD and all-cause dementia in men with BPH compared with men from the general population was not explained by BPH treatment or by underlying diabetes, cardiovascular disease, or chronic kidney disease. We did not directly assess sleep disturbances, but the higher risksupport the hypothesis that sleep disturbances are a risk factor for AD and perhaps other causes of dementia. If confirmed, the diagnosis of BPH may present an opportunity to reduce dementia risk in men by treatments apart from anticholinergic drugs that reduce sleep-disruptive symptoms. Such treatments might include sleep cognitive behavioral therapy, reducing alcohol and fluid intake before bedtime, taking diuretics earlier in the day, and elevating the legs in the evening to reduce dependent edema.
Contributors
MN designed the study, classified register data, interpreted study results, and drafted the manuscript. EHP contributed to the study design; planned the statistical analyses; obtained, linked, and analyzed the data; interpreted the study results; and revised the manuscript. PC contributed to the study design, interpreted the study results, and revised the manuscript. HTS conceived the study, obtained the data, interpreted the study results, and revised the manuscript. VWH conceived the study, contributed to planning the statistical analyses, interpreted the study results, and revised the manuscript. MN and HTS are guarantors of the paper. MN, HTS, and EHP had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Data sharing
Due to Danish legislation, the individual‐level data used in the study cannot be made available to other researchers as these are kept on a secured server at the Danish Health Data Authority.
Funding
Lundbeckfonden, Aarhus University Research Foundation, and the National Institutes of Health.
Declaration of Competing Interests
Dr. Sørensen and Dr. Nørgaard report grants from Lundbeckfonden (grant no. R248–2017–521) and by the Aarhus University Research Foundation. VWH was supported by a grant from the Alzheimer center (National Institutes of Health grant P50 AG047366). All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare no other support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgment
The study was supported by Lundbeckfonden, and by the Aarhus University Research Foundation. None of the study sponsors had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The researchers acted independently from the study sponsors in all aspects of this study.
Contributor Information
Mette Nørgaard, Email: mn@clin.au.dk.
Erzsébet Horváth-Puhó, Email: ep@clin.au.dk.
Henrik Toft Sørensen, Email: hts@clin.au.dk.
Victor W. Henderson, Email: vhenderson@stanford.edu.
Appendix
International Classification of Diseases (ICD) and other codes used in the study.
| ICD-8 | ICD-10 | |
| BPH | 600 | N40 |
| Transurethral resection of the prostate (TURP) | Surgical code 55,080 | Surgical code KKED22 |
| Medical treatment for BPH: | ||
| Alpha-receptor blockers | ATC-codes:C02CA and G04CA | |
| 5-alpha reductase inhibitors | ATC-code:G04CB | |
| Underlying comorbidities | ||
| Cardiovascular disease: | ||
| Myocardial infarction | 410 | I21 |
| Heart failure | 427.09–427.11, 427.19, 428.99, 782.49 | I50, I11.0, I13.0, I13.2 |
| Atrial fibrillation or flutter | 427.93–94 | I48 |
| Stroke and subarachnoid hemorrhage |
430–436 |
I60–64 |
| Aortic diseases including intermittent claudication |
440–445 |
I70-I74, I77 |
| Diabetes mellitus | 249–250 | E10, E11 |
| Hyperlipidemia and hypercholesterolemia | 272.00–09, 279.00, 279.01 | E78.0-E78.2, E78.4, E78.5 |
| Chronic obstructive pulmonary disease | 490–493, 515–518 | J40-J47, J60-J67, J58.4, J70.1 |
| Hypertension | 400, 401 | I10-I11, |
| Obesity | 277 | E65-E68 |
| Renal failure | 79,299, 58,499 | N18, N19, N13.8–9 |
| Prostate cancer | 185.99 | C61 |
| Outcomes | ||
| Alzheimer's disease | 290.10, 290.09 | F00, G30 |
| Other dementias | 094.19 and 292.09, 290.11, 290.18, 290.19, 293.09, 293.19 | F01, F02, F03, F1x.73 series, G23.1, G31.0, G31.1, G31.8B, G31.8E, G31.85 |
| Diagnoses that might indicate early symptoms of dementia (mild cognitive impairment and amnestic syndromes) | 291.19 |
F04, F04.9, F05.1, F06.7, F10.6, F18.6, F19.6 |
| Prescription medicine | ||
| Antihypertensive drugs | ATC-code: C02 (except C02CA), C03, C04, C07, C09 | |
| Nonsteroidal Anti-inflammatory Drugs | ATC-code: M01A | |
| Testosterone | ATC-code: G03B (ATC code) or Treatment code: BBHG1 |
|
References
- 1.Alzheimer's disease facts and figures Alzheimer's. Dementia. 2020;16(3):391. doi: 10.1002/alz.12068. [DOI] [PubMed] [Google Scholar]
- 2.Aisen P.S., Cummings J., Jack C.R., Jr. On the path to 2025: understanding the Alzheimer's disease continuum. Alzheimers Res Ther. 2017;9(1):60. doi: 10.1186/s13195-017-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarasoff-Conway J.M., Carare R.O., Osorio R.S. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11(8):457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliwise D.L., Foley D.J., Vitiello M.V., Ansari F.P., Ancoli-Israel S., Walsh J.K. Nocturia and disturbed sleep in the elderly. Sleep Med. 2009;10(5):540–548. doi: 10.1016/j.sleep.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshimura K., Ohara H., Ichioka K. Nocturia and benign prostatic hyperplasia. Urology. 2003;61(4):786–790. doi: 10.1016/s0090-4295(02)02444-5. [DOI] [PubMed] [Google Scholar]
- 6.Lee S.W.H., Chan E.M.C., Lai Y.K. The global burden of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a systematic review and meta-analysis. Sci Rep. 2017;7(1):7984. doi: 10.1038/s41598-017-06628-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt M., Pedersen L., Sorensen H.T. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M., Schmidt S.A., Sandegaard J.L., Ehrenstein V., Pedersen L., Sorensen H.T. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mors O., Perto G.P., Mortensen P.B. The Danish psychiatric central research register. Scand J Public Health. 2011;39(7 Suppl):54–57. doi: 10.1177/1403494810395825. [DOI] [PubMed] [Google Scholar]
- 10.Pottegard A., Schmidt S.A.J., Wallach-Kildemoes H., Sorensen H.T., Hallas J., Schmidt M. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2017;46(3) doi: 10.1093/ije/dyw213. 798-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nygard L.H., Talala K., Taari K., Tammela T.L.J., Auvinen A., Murtola T.J. The effect of non-steroidal anti-inflammatory drugs on risk of benign prostatic hyperplasia. Prostate. 2017;77(9):1029–1035. doi: 10.1002/pros.23359. [DOI] [PubMed] [Google Scholar]
- 12.Liao C.H., Li H.Y., Chung S.D., Chiang H.S., Yu H.J. Significant association between serum dihydrotestosterone level and prostate volume among Taiwanese men aged 40-79 years. Aging Male:Off J Int Soc Study Aging Male. 2012;15(1):28–33. doi: 10.3109/13685538.2010.550660. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C., Wang Y., Wang D., Zhang J., Zhang F. NSAID exposure and risk of Alzheimer's disease: an updated meta-analysis from cohort studies. Front Aging Neurosci. 2018;10:83. doi: 10.3389/fnagi.2018.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv W., Du N., Liu Y. Low testosterone level and risk of Alzheimer's disease in the elderly men: a systematic review and meta-analysis. Mol Neurobiol. 2016;53(4):2679–2684. doi: 10.1007/s12035-015-9315-y. [DOI] [PubMed] [Google Scholar]
- 15.Petersson F., Baadsgaard M., Thygesen L.C. Danish registers on personal labour market affiliation. Scand J Public Health. 2011;39(7 Suppl):95–98. doi: 10.1177/1403494811408483. [DOI] [PubMed] [Google Scholar]
- 16.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chughtai B., Forde J.C., Thomas D.D. Benign prostatic hyperplasia. Nat Rev Dis Prim. 2016;2:16031. doi: 10.1038/nrdp.2016.31. [DOI] [PubMed] [Google Scholar]
- 18.Morris J.K., Uy R.A.Z., Vidoni E.D. Effect of APOE epsilon4 genotype on metabolic biomarkers in aging and Alzheimer's disease. J Alzheimer's Dis: JAD. 2017;58(4):1129–1135. doi: 10.3233/JAD-170148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gacci M., Corona G., Sebastianelli A. Male lower urinary tract symptoms and cardiovascular events: a systematic review and meta-analysis. Eur Urol. 2016;70(5):788–796. doi: 10.1016/j.eururo.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Sundboll J., Hovath-Puho E., Adelborg K. Higher risk of vascular dementia in myocardial infarction survivors. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.117.029127. [DOI] [PubMed] [Google Scholar]
- 21.Rule A.D., Jacobson D.J., Roberts R.O. The association between benign prostatic hyperplasia and chronic kidney disease in community-dwelling men. Kidney Int. 2005;67(6):2376–2382. doi: 10.1111/j.1523-1755.2005.00344.x. [DOI] [PubMed] [Google Scholar]
- 22.Drew D.A., Weiner D.E., Sarnak M.J. Cognitive impairment in CKD: pathophysiology, management, and prevention. Am J Kidney Dis: Off J Natl Kidney Found. 2019 doi: 10.1053/j.ajkd.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koseoglu H., Aslan G., Ozdemir I., Esen A. Nocturnal polyuria in patients with lower urinary tract symptoms and response to alpha-blocker therapy. Urology. 2006;67(6):1188–1192. doi: 10.1016/j.urology.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Jessen N.A., Munk A.S., Lundgaard I., Nedergaard M. The glymphatic system: a beginner's guide. Neurochem Res. 2015;40(12):2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tae B.S., Jeon B.J., Choi H., Cheon J., Park J.Y., Bae J.H. Alpha-blocker and risk of dementia in patients with benign prostatic hyperplasia: a nationwide population based study using the national health insurance service database. J Urol. 2019;202(2):362–368. doi: 10.1097/JU.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 26.Lin H.J., Weng S.F., Yang C.M., Wu M.P. Risk of hospitalization for acute cardiovascular events among subjects with lower urinary tract symptoms: a nationwide population-based study. PLoS One. 2013;8(6):e66661. doi: 10.1371/journal.pone.0066661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kupelian V., Rosen R.C., Link C.L. Association of urological symptoms and chronic illness in men and women: contributions of symptom severity and duration–results from the BACH Survey. J Urol. 2009;181(2):694–700. doi: 10.1016/j.juro.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ott A., Slooter A.J., Hofman A. Smoking and risk of dementia and Alzheimer's disease in a population-based cohort study: the Rotterdam study. Lancet. 1998;351(9119):1840–1843. doi: 10.1016/s0140-6736(97)07541-7. [DOI] [PubMed] [Google Scholar]
- 29.Xu H., Fu S., Chen Y., Chen Q., Gu M., Wang Z. Smoking habits and benign prostatic hyperplasia: a systematic review and meta-analysis of observational studies. Medicine (Baltimore) 2016;95(32):e4565. doi: 10.1097/MD.0000000000004565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dmochowski R.R., Thai S., Iglay K. Increased risk of incident dementia following use of anticholinergic agents: a systematic literature review and meta-analysis. Neurourol Urodyn. 2020 doi: 10.1002/nau.24536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowles E.J.A., Walker R.L., Anderson M.L., Dublin S., Crane P.K., Larson E.B. Risk of Alzheimer's disease or dementia following a cancer diagnosis. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0179857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montorsi F., Mercadante D. Diagnosis of BPH and treatment of LUTS among GPs: a European survey. Int J Clin Pract. 2013;67(2):114–119. doi: 10.1111/j.1742-1241.2012.03012.x. [DOI] [PubMed] [Google Scholar]