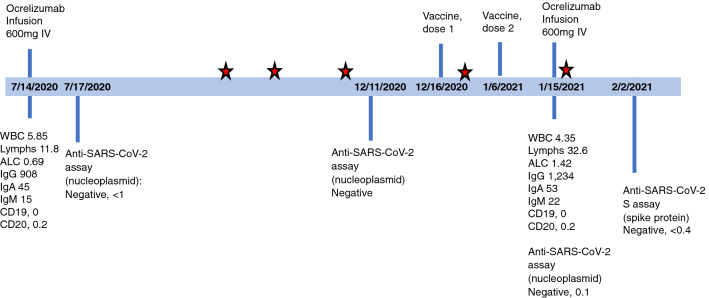
Fig. 1.
Timeline of patient’s ocrelizumab infusions, vaccination, SARS-CoV-2 test and laboratory results. Patient had serial SARS-CoV-2 PCR tests (negative) through the institutional research program. The red stars indicate negative SARS-CoV-2 PCR tests. Reference range for Anti-SARS-CoV-2 assay (nucleoplasmid) < 1.00 COI, Anti-SARS-CoV-2 spike (S) < 0.8 U/mL, WBC 4.00–10.00 K/uL, Lymphocytes 18.0–41.0%, ALC 0.72–4.10 K/uL, IgG 700–1,600 mg/dL, IgA 70–400 mg/dL, IgM 40–230 mg/dL, CD19 7.0–27.0% lymphocytes, CD20 3.0–20.0% lymphocytes