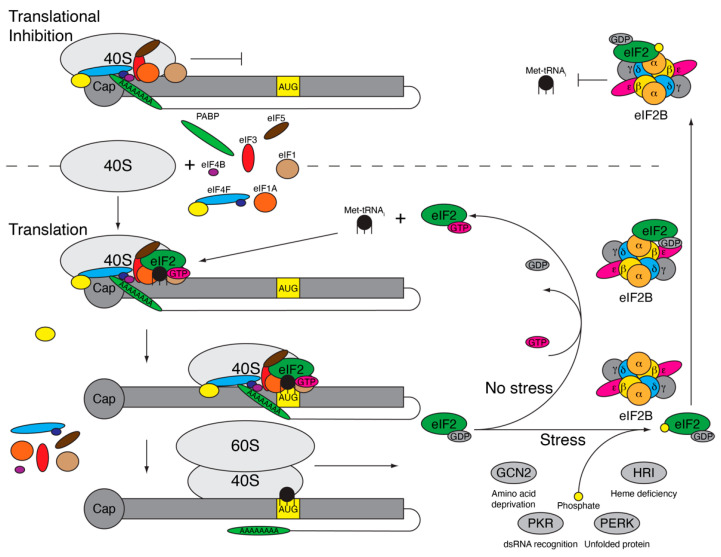
Figure 1.
Phosphorylation of eukaryotic initiation factor-2 (eIF2α) results in translational inhibition. Under normal conditions (bottom), the 40S ribosomal subunit and initiation factors eIF1, eIF1A, eIF3, eIF4B, eIF4F complex, and eIF5 come together along with the ternary complex (eIF2, guanosine triphosphate [GTP], and initiator methionine transfer RNA [Met-tRNAi}) to form the 48S preinitiation complex (PIC). The 48S PIC begins scanning for the start codon, AUG, in the correct context, resulting in GTP hydrolysis and eIF2–GDP release along with the other initiation factors. The 60S subunit binds with the 40S subunit and translation elongation begins. eIF2–GDP binds eIF2B, which exchanges GDP for GTP, allowing eIF2–GTP to bind another Met-tRNAi, form the 48S PIC, and initiate translation again. During stress, eIF2α is phosphorylated by eIF2α kinases (general control non-depressible 2 (GCN2), protein kinase R (PKR), PKR-like endoplasmic reticulum kinase (PERK), or heme-regulated inhibitor (HRI)), triggering eIF2–GDP to get stuck binding eIF2B within the nonproductive site, inhibiting the exchange of GDP for GTP. The 48S PIC, missing ternary complex (48S* PIC), cannot initiate translation and becomes stuck at the 5′ end (top), resulting in ribonucleoprotein complex accumulation and stress granule formation.