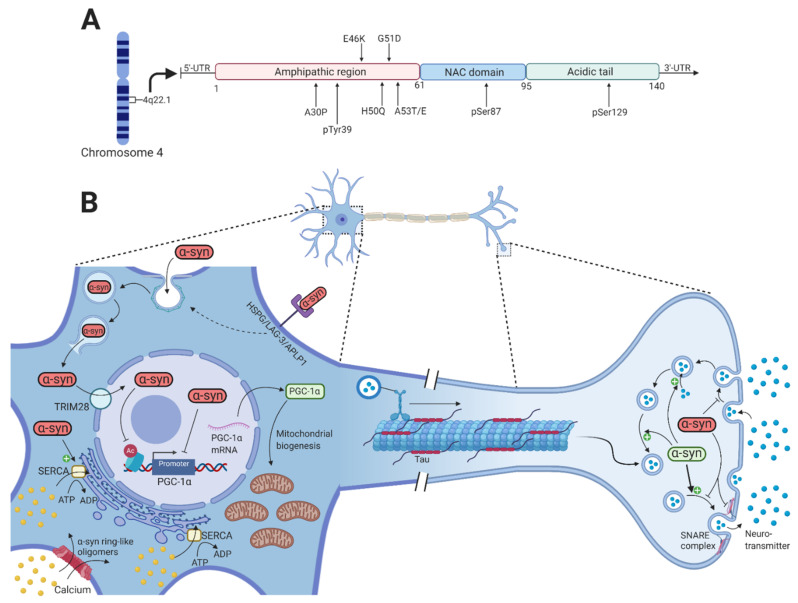
Figure 1.
-synuclein function and pathology. (A) Shows a schematic representation of -synuclein on transcript level with color-coded functional domains and mutations of -synuclein mentioned in this article. (B) Under physiological conditions, -synuclein (green) is involved in the compartmentalization, storage, and recycling of neurotransmitters. It also contributes to the exocytotic process by promoting SNARE-complex assembly, thereby enabling the fusion of intracellular presynaptic vesicles with the presynaptic membrane. Neurotoxic alterations of -synuclein (red) by missense mutations in the SNCA gene, gene duplication or triplication, and various PTMs increase the formation of toxic oligomers and fibrils that disrupt intracellular processes. The internalization of toxic -synuclein is through dynamin-mediated endocytosis and cell surface protein-mediated uptake through HSPG, LAG-3, and APLP1. Inside the endosome, -synuclein is able to rupture the membrane to allow direct entry to the cytosol. -synuclein oligomers can permeabilize the plasma membrane by creating pore-like structures, thus increasing the cytosolic calcium concentration. Activation of the SERCA pump by these oligomers will later contribute further to the increased cytosolic calcium. Through TRIM28, toxic -synuclein accumulates inside the nucleus where it binds and inhibits the PGC-1 promoter and reduces histone H3 acetylation, potentially affecting numerous cellular processes. This results in reduced PGC-1 mRNA and protein, which reduces mitochondrial biogenesis. Created with BioRender.com.