Table 2.
Characteristics of AVTs, antibiotics and vaccines *.
| Characteristics | AVTs | Antibiotics | Hypothetical Vaccine |
|---|---|---|---|
| Mode of action | Selective inhibition of pathogens, preserves the microbiome | Broad spectrum killing of microorganisms, removes the microbiome | Selective inhibition of pathogen, preserves the microbiome |
| Mechanism of action | Tailored to prevent colonization, transmission, and infection by a pathogen | Kills systemic microorganisms—resolves acute infections. Not used for asymptomatic infections | Prevents acute infection by a pathogen. In some instances, vaccines can prevent colonization and transmission of the pathogen. |
| Use | Pre-exposure prophylaxis or therapeutic | Therapeutic | Pre-exposure therapeutic |
| Dose | Multiple dosing as needed | Multiple dosing, 3–4 days | 1–3 doses |
| Route of administration | Oral, topical
|
Oral, injectable
|
Injectable
|
| Correlate of protection | Absence of viable pathogen at the site of infection | Absence of viable pathogen at the site of infection | Currently unknown |
| Implementation | Pharmacy or medical prescription | Medical prescription | Primary care clinics |
| Size and cost of clinical trials | >1000 subjects ** <$1 billion |
>10,000 subjects >$1.5 billion |
>100,000 subjects >$1.8 billion |
| Drug development timeframe from pre-clinical to licensing | 5–10 years ** | 9–15 years | 9–15 years |
| Licensing pipeline |
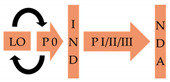
|
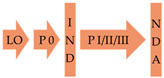
|
|
* Information tabulated from Paul et al. (2010) [294] and Farha and Brown (2019) [291]. ** Provisional estimates as the licensing pipeline has not been fully established for AVTs against N. gonorrhoeae. LO: Lead optimization. P 0: Phase 0. P I/II/III: Phase I/II/III. IND: investigational new drug application. NDA: new drug application. FDA: United States Food and Drug Administration (FDA) review and approval.
