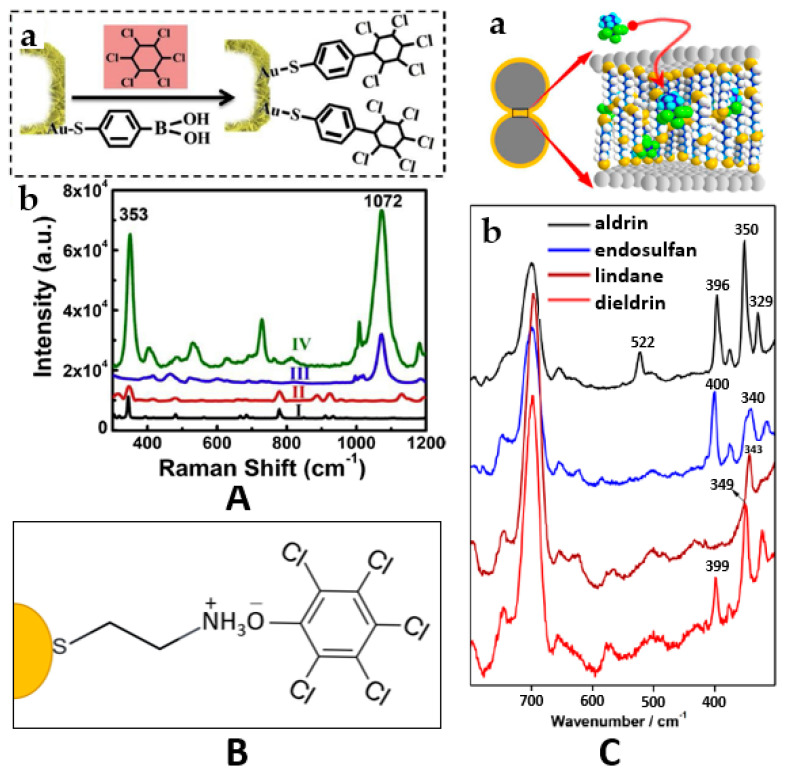
Figure 3.
Strategies for increasing the affinity toward the analyte. (A) (a) The Suzuki cross coupling reaction occurred between HCH and 4-MPBA via covalent linkage (C–C) on the modified substrate. (b) The Raman spectra of solid HCH (curve I), the SERS spectra of γ-HCH (10−6 M) obtained on the naked ANHC substrate (curve II), 4-MPBA modified ANHC substrate (curve III), 4-MPBA modified ANHC substrate immersed into 10−6 M HCH solution (curve IV). Reproduced with permission of [53]. Copyright Elsevier, 2019. (B) Schematic representation of the mechanism of sensing PCP using cysteamine modified substrates. (C) (a) Scheme displaying the pesticide hosting (in this case, for aldrin) in the dithiol layer organized in interparticle gaps; (b) SERS spectra of the analyzed pesticides (10−5 M) on DT8-functionalized AgNPs, showing the C–Cl stretching bands of the fingerprint region (300–400 cm−1) and the C–S stretching bands of DT8 in the deduced multilayer highly ordered conformation employed as the reference band for quantitative analysis. Reproduced with permission of [23]. Copyright American Chemical Society, 2015.