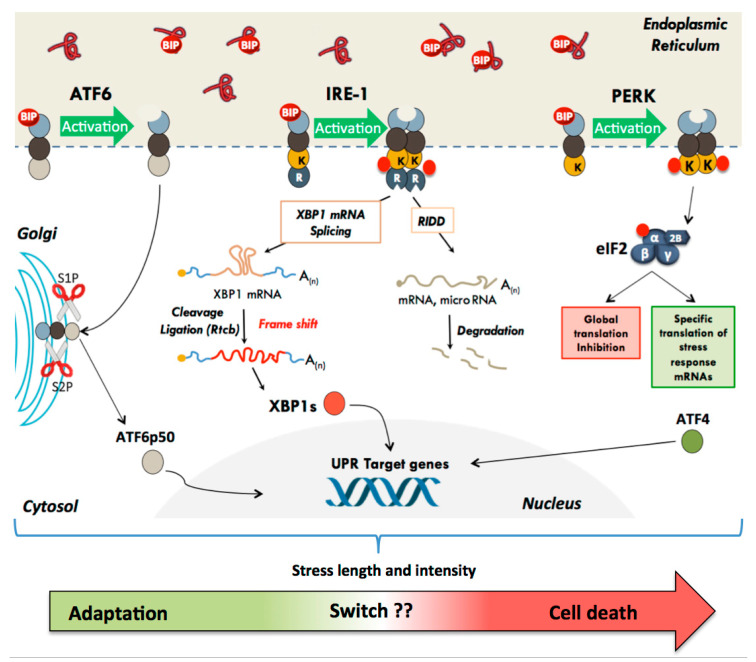
Figure 1.
The different UPR effectors and their modes of action. In the basal state, the three UPR effector transmembrane proteins (PERK, ATF6, and IRE-1) are maintained inactive through their interaction with the protein chaperone BiP. The accumulation of misfolded proteins in the ER lumen results in dissociation of BiP and activation of UPR. (1) PERK dimerizes and phosphorylates the eIF2α subunit, leading to a global inhibition of translation initiation. Specific mRNA subsets, containing cis-acting elements in their 5′UTR, such as uORF and IRES, escape translational inhibition triggered by eIF2 phosphorylation. (2) IRE-1 initiates an unconventional splicing of XBP-1 mRNA. IRE1α cleaves Xbp1u mRNA within two stem-loop structures, leading to excision of 26 nucleotides. Subsequent ligation of the Xbp1 mRNA by the tRNA ligase RTCB results in a frame shift and allows the translation of the active transcription factor XBP1s, which is imported into the nucleus and activates the expression of target genes. IRE1α mediates also the degradation of some RNAs (this mechanism has been called RIDD for Regulated Ire1-Dependent Decay). (3) BIP dissociation from ATF6 exposes its Golgi Localization Signal. ATF6 is translocated to the Golgi apparatus where proteolysis releases its transcription factor amino-terminal domain, which is imported into the nucleus and activates the expression of target genes. The UPR has a primary function in adaptive response in order to restore homeostasis and promote cell survival, but depending on the duration and intensity of the stress, a switch can induce cell death to get rid of the damaged cells.