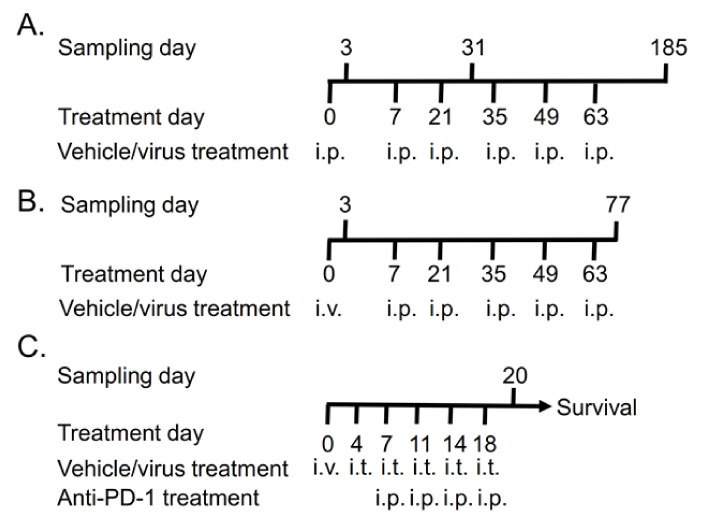
Figure 1.
Treatment schedule for TILT-123 safety studies: (A) 15 male and female hamsters received saline or 1 × 1010, 1 × 1011, or 1 × 1012 virus particles (VP)/kg of TILT-123 six times intraperitoneally (i.p.). Five animals/sex/group were sacrificed 3, 31, and 185 days after the first treatment. (B) Ten male and female hamsters received saline, 1 × 1011 VP/kg, or 1 × 1012 VP/kg of TILT-123 once intravenously (i.v.) and five times i.p. Five animals/sex/group were sacrificed 3 days after the first injection and two weeks after the last injections (on day 77). (C) Human fibrosarcoma tumors were implanted subcutaneously to both flanks of nude NMRI mice. Nine days after implantation, the animals received 1 × 105 VP, 1 × 107 VP, or 1 × 109 VP i.v. The following treatments were injected intratumorally (i.t.) Anti-PD-1 (100 µg) treatments were started on day 7 after two virus treatments. Five animals per group were sacrificed on day 20, and the rest (6–7 animals/group) continued in the survival study.