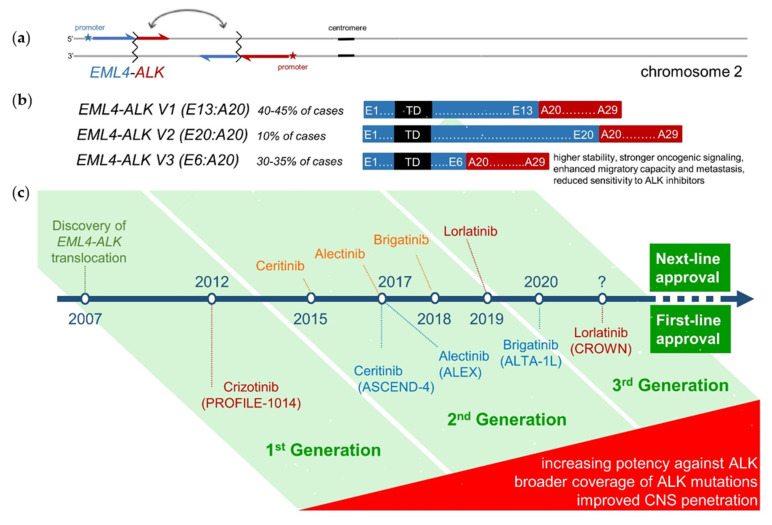
Figure 1.
(a) Molecular pathogenesis of ALK+ NSCLC: In approximately 90% of cases, a paracentric inversion in the short arm of chromosome 2 [inv(2)(p21p23)] creates the fusion oncogene EML4-ALK, which facilitates overexpression of the ALK exons 20–29 under the influence of the EML4 promoter, and their autophosphorylation due to the trimerization domain (TD) of EML4 [6]. In the remaining 10% of cases, a different partner gene is fused with ALK and triggers similar pathophysiologic effects. (b) The length of the EML4 component can differ between patients, with 90% harboring EML4-ALK fusion variant 1, 2 or 3. Shorter variants (mainly variant 3, aka V3, E6:A20, present in 30–35% of cases) have higher stability, accumulate more and interact better with the cytoskeleton, causing stronger oncogenic signaling, reduced sensitivity to ALK inhibitors, enhanced migration and metastasis [20,21,22,23,24]. (c) Approval timeline of currently available ALK inhibitors, along with their respective first-line phase 3 trials. Next-generation ALK inhibitors show higher potency against the native ALK oncoprotein, broader coverage against ALK resistance mutations, and improved CNS penetration [7,8,9].