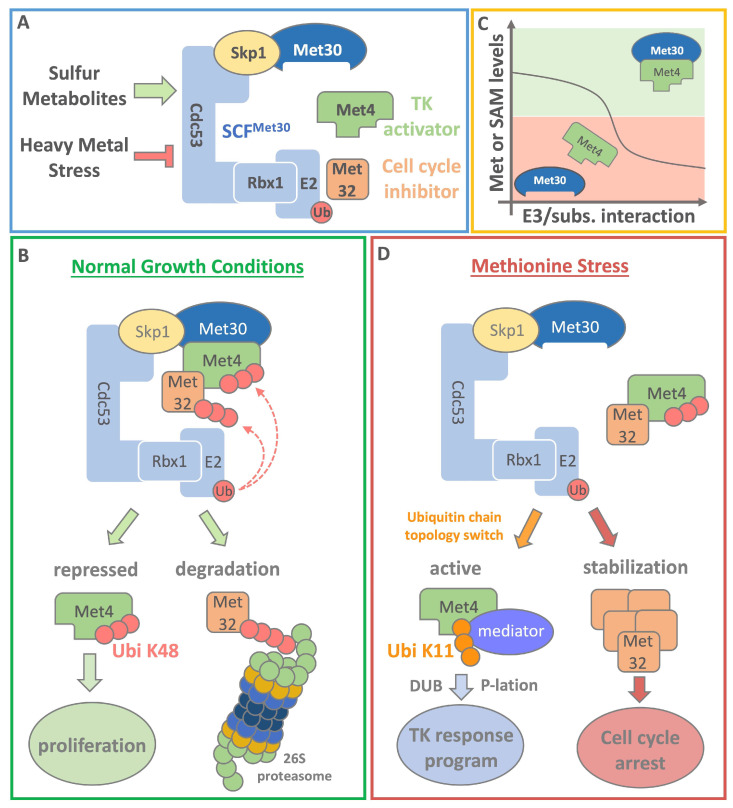
Figure 2.
Methionine perception in yeast. (A) Blue box: The E3 ubiquitin ligase SCFMet30 is the center of the response to methionine starvation and heavy metal (cadmium) stress in yeast. The most critical substrates of the E3 ligase are the transcriptional activator Met4 and the cell cycle inhibitor Met32. Together they orchestrate the transcriptional response program during nutritional or heavy metal stress, however through profoundly distinct mechanisms. (B) Green box: Under normal growth conditions Met4 is ubiquitinylated by SCFMet30. The attached K48 linked ubiquitin chain does not lead to the degradation of Met4. Instead, Met4 is kept in a stable and transcriptionally inactive state. Met4 is also a substrate receptor for the cell cycle inhibitor Met32. Met4 recruits Met32 to SCFMet30 to catalyze the ubiquitylation and subsequent degradation of the cell cycle inhibitor via the 26S proteasome pathway to ensure proliferation. (C) Yellow box: Under normal growth conditions, high levels of methionine and metabolites promote a stable Met4 complex with the E3 ligase via the F-box protein Met30. Low methionine or SAM levels lead to the dissociation of SCFMet30 from its substrate Met4. (D) Red box: Under methionine or SAM starvation conditions, a change in Met4 ubiquitin chain topology is induced from K48 to K11 linkage, allowing the association of the transcriptional coactivator complex mediator with Met4. After this initiation step, Met4 is deubiquitylated (DUB) and phosphorylated (P-lation). The now active Met4 drives starts a transcriptional (TK) response program that drives genes to restore SAM levels. Dissociation of Met4 in turn also blocks ubiquitylation of Met32, leading to its stabilization. Accumulation of Met32 triggers a cell cycle arrest. Once sulfur containing metabolites are restored binding between SCFMet30 and its substrates is promoted. Met4 and Met32 get re-ubiquitylated resulting in transcriptional inactivation and degradation respectively. The cell cycle arrest is terminated and proliferation can progress.