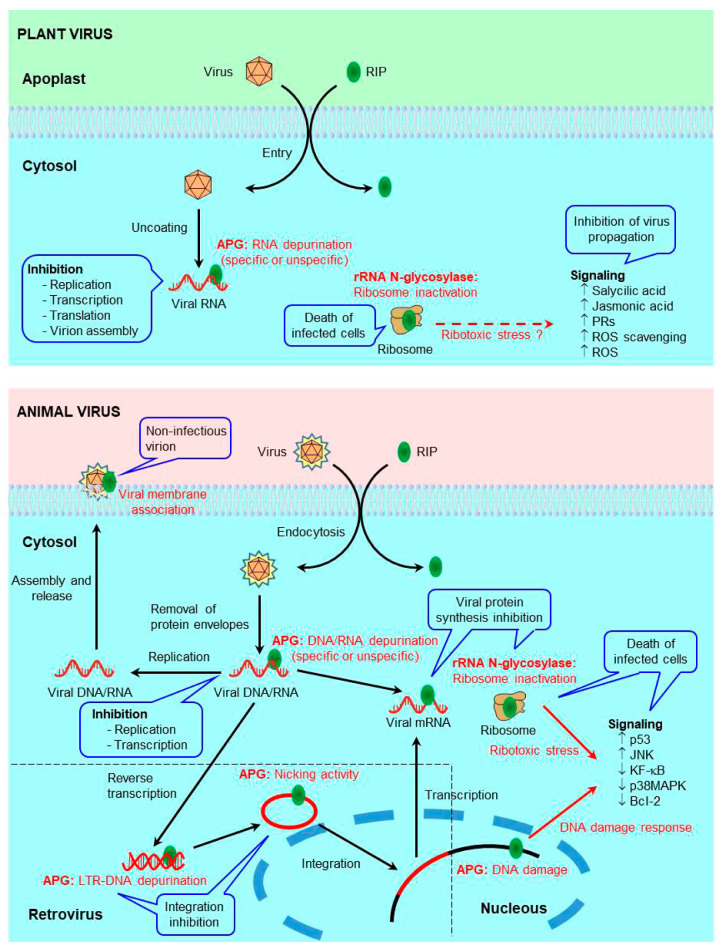
Figure 1.
Proposed mechanisms for the antiviral activity of RIPs against plant viruses (upper panel), animal viruses (lower panel), and retroviruses (lower panel including dashed square). (upper panel) In plants, viral infection promotes the passage of the RIP from the apoplast to the cytosol. In the cytosol, it can inactivate ribosomes (rRNA glycosylase activity), causing the death of infected cells and thus preventing the spread of the virus. The RIP can also depurinate the viral RNA (adenine polynucleotide glycosylase, APG, activity), inhibiting its replication, transcription, translation, and assembly. It can also trigger antiviral defense signaling pathways, causing an increase in the levels of salicylic acid, jasmonic acid, pathogenesis-related (PR) proteins, and both reactive oxygen species (ROS) and ROS scavenging enzymes. (lower panel) In animal cells, the RIP can enter by pinocytosis or receptor-mediated endocytosis. RIP can inactivate ribosomes (rRNA glycosylase activity), causing the death of infected cells or inactivate the viral genome, DNA, or RNA (APG activity), preventing their replication, transcription, and translation. Some RIPs depurinate specific sequences (APG activity), blocking critical functions for the virus life cycle. In the case of retroviruses, the RIP can also depurinate the long terminal repeats (LTRs) (APG activity) or cleave the circular DNA (APG activity) preventing its integration into the cell genome. It can also be introduced into virions during budding (viral membrane association), making them less infective. Ribotoxic stress (rRNA glycosylase activity or APG activity on mRNA) and DNA damage (APG activity) caused by RIPs can trigger the activation of signaling pathways that cause infected-cell death preventing virus spreading.