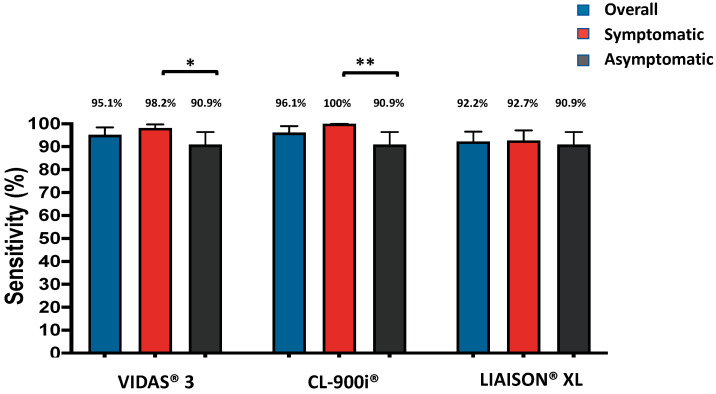
Figure 4.
Sensitivity for each assay on samples collected ≥ 21 days post symptom onset in patients with SARS-CoV-2 RT-PCR-confirmed infection using the sVNT as a reference test. Data are presented for 111 RT-PCR confirmed SARS-CoV-2 positive samples categorized as: overall (n = 111), symptomatic (n = 56); and asymptomatic (n = 51); run on each automated assay; VIDAS®3, CL-900i® SARS-CoV-2, and LIAISON®XL. Chi-square test was used to detect the presence of a statistically significant difference in the sensitivity of each assay between the symptomatic and asymptomatic samples. * p < 0.05, ** p < 0.001.