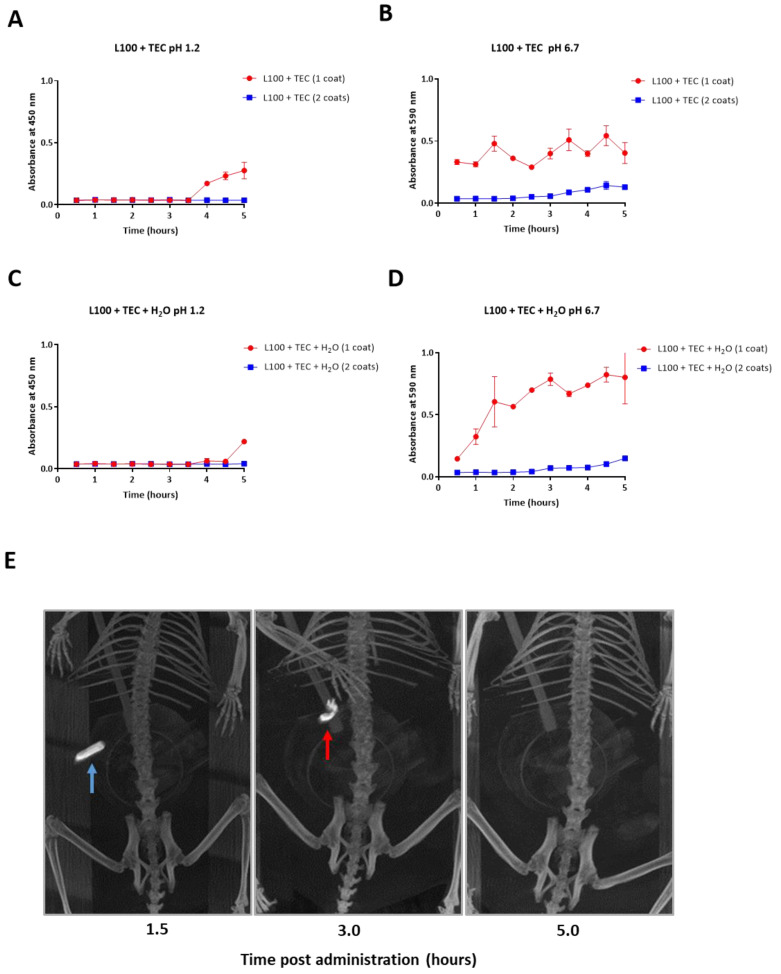
Figure 3.
Dissolution of coated capsules in different pH conditions in vitro (A–D) and in vivo (E) to establish the optimal enteric coating required for targeted release of formulation in the small intestine. An in vitro assay was conducted to compare enteric capsules dip coated once or twice in EUDRAGIT® L100 in 10% TEC without the addition of 3% H20 (A,B) and with 3% H20 (C and D). Dissolution of the capsules was tested in simulated gastric fluid pH 1.2 over 5 h (A,C) then in stimulated intestinal fluid pH 6.7 over 5 h (B,D) by measuring the changing absorbance of surrounding solutions over time due to release of Bromophenol Blue from the capsules. Error bars represent standard error of the mean (SEM). (E) Representative CT images in one hamster out of 4, showing localisation and dissolution of optimally coated capsule containing BaSO4 over time after oral administration. The capsule is found intact at the level of the stomach at 1.5 h (blue arrow) indicating stability of the coated capsule at the low stomach pH. At 3 h the capsule is entering the small intestine (red arrow) and is disintegrating at the higher intestinal pH. At 5 h the capsule has completely disintegrated, and its contents released and fully solubilised.