Fig. 2 |. Intrinsic and extrinsic apoptosis pathways.
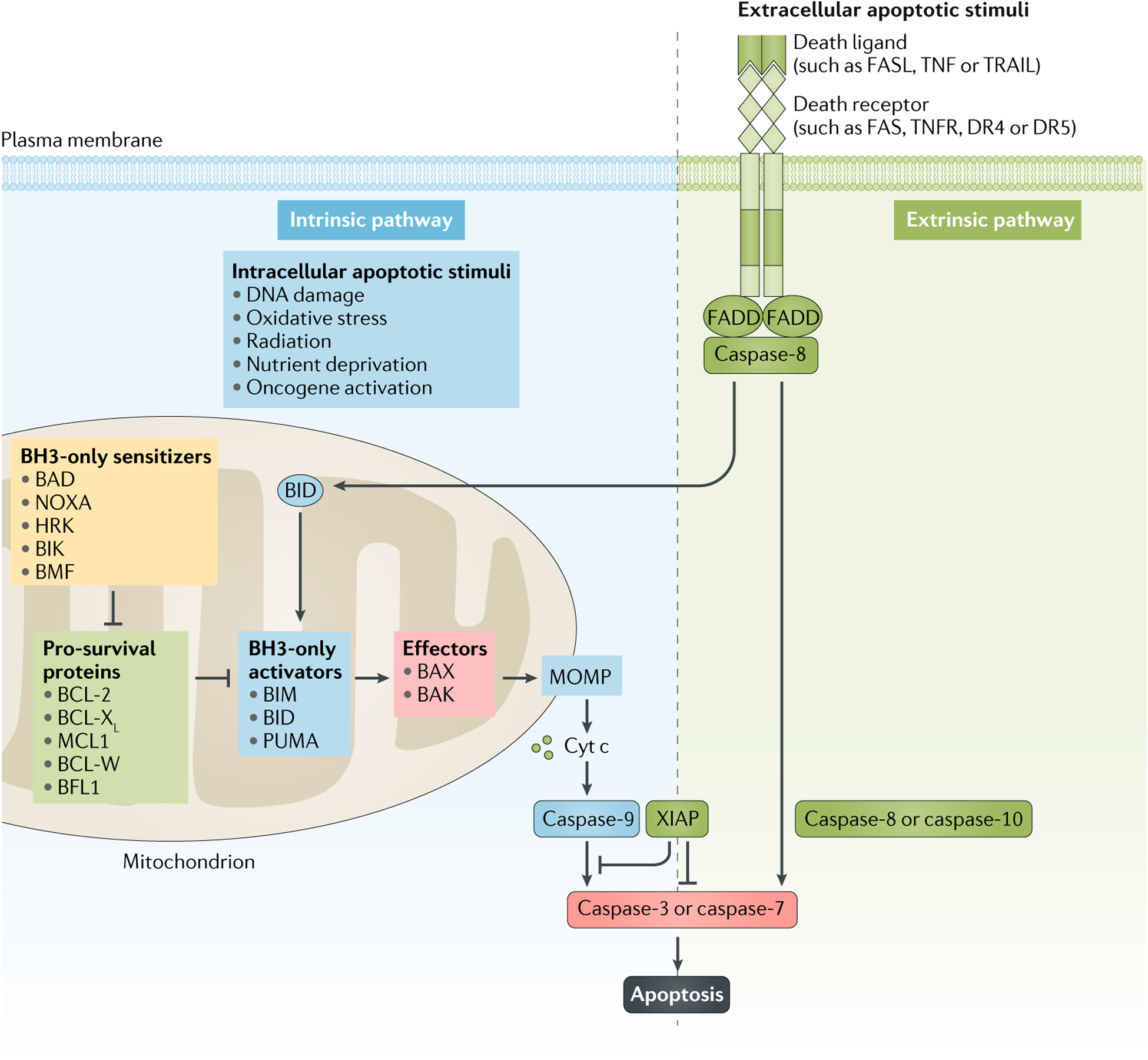
Apoptosis is controlled by two distinct yet connected pathways. The intrinsic pathway is triggered by intracellular death stimuli, such as DNA damage, radiation, nutrient deprivation, oxidative stress or oncogene activation, which induce apoptosis by promoting mitochondrial outer membrane permeabilization (MOMP) and cytochrome c (cyt c)-dependent activation of caspase-9, which then activates caspase-3 and caspase-7. This pathway of apoptosis is tightly controlled by the BCL-2 family of proteins, which are classified on the basis of their structural homology and function into sensitizers, pro-survival proteins, activators and effectors. MOMP is controlled by homo-oligomerization of the effector proteins BCL-associated X protein (BAX) and BCL-2 homologous antagonist/killer (BAK), which are activated by the activator proteins BCL-2-like protein 11 (BCL2L11; also known as BIM), p53-upregulated modulator of apoptosis (PUMA) and BH3-interacting domain death agonist (BID). Pro-survival proteins such as BCL-2, BCL-XL, BCL-W, induced myeloid leukaemia cell differentiation protein MCL1 (MCL1) and BCL-2-related protein A1 (BCL2A1; also known as BFL1) can bind and sequester both activators and effectors, thereby preventing their interaction and the induction of MOMP. Sensitizer proteins (such as BCL-2-associated death promoter (BAD), BCL-2 interaction killer (BIK), BCL-2 modifying factor (BMF), activator of apoptosis harakiri (HRK) and phorbol-12-myristate-13-acetate-induced protein 1 (PMAIP1; also known as NOXA) are a distinct group of proteins that promote apoptosis by binding and blocking pro-survival proteins, thereby releasing formerly bound activator and effector proteins. The extrinsic pathway is initiated by extracellular ligands such as FAS ligand (FASL), TNF and TNF ligand superfamily member 10 (TNFSF10; also known as TRAIL), which bind to the cell-surface receptors FAS, TNF receptors (TNFRs) and death receptor 4 (DR4) and DR5, respectively. The signal is transmitted via FAS-associated death domain (FADD), which triggers the activation of caspase-8 and caspase-10, ultimately initiating apoptosis through cleavage and activation of pro-caspase-3 and pro-caspase-7. BH3, BCL-2 homology domain 3; XIAP, X-linked inhibitor of apoptosis protein.
