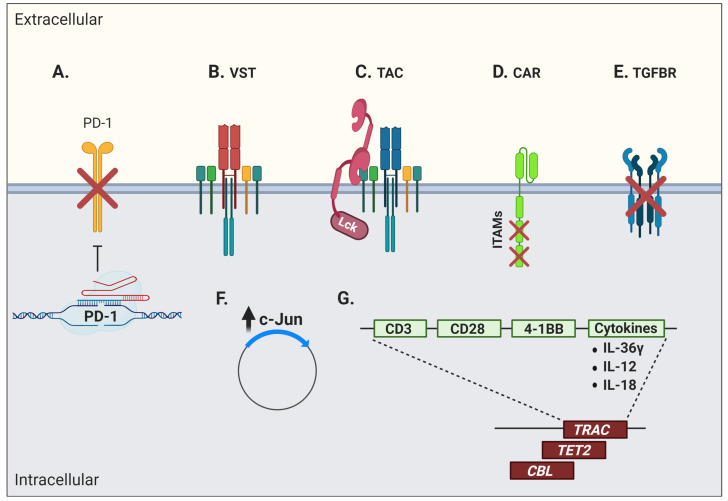
Figure 3.
Interventions aiming at limiting T-cell dysfunction in engineered cells for adoptive immunotherapy. Engineered cells comprise transgenic T-cell receptor (TCR) and chimeric/synthetic antigen receptor T cells. (A) Genetic ablation of negative co-signaling molecules (e.g., PD-1) may be used to circumvent the effects of T-cell dysfunction. (B) Virus-specific T cells (VSTs) have been evaluated as a platform for artificial receptor expression in order to leverage their robust memory differentiation. (C) Another strategy to increase modified T-cell fitness is the use of a T-cell antigen coupler (TAC) to avoid tonic signaling and leverage endogenous TCR signaling. (D) Advanced engineering of CAR molecules involving the inactivation of two out of three ITAM motifs or (E) the blockade of suppressive cytokine signaling, here shown by the overexpression of a dominant negative TGFβ receptor, can also promote better T-cell functions. (F) An increase in c-Jun activity has been shown to further limit tonic signaling and T-cell dysfunction. (G) Disruption of the endogenous TCRα/β chains by CRISPR/Cas9 or specific integration of the CAR vector into the targeted DNA locus (e.g., TRAC) have shown additional efficacy. This was also true when CAR constructs inadvertently disrupted the TET2 and CBL genes. Other strategies include the enhancement of T-cell function through the production of stimulatory cytokines (e.g., IL-36γ, IL-12, and IL-18) or co-stimulatory molecules (e.g., CD28 and 4-1BB). This figure was created with BioRender.com.