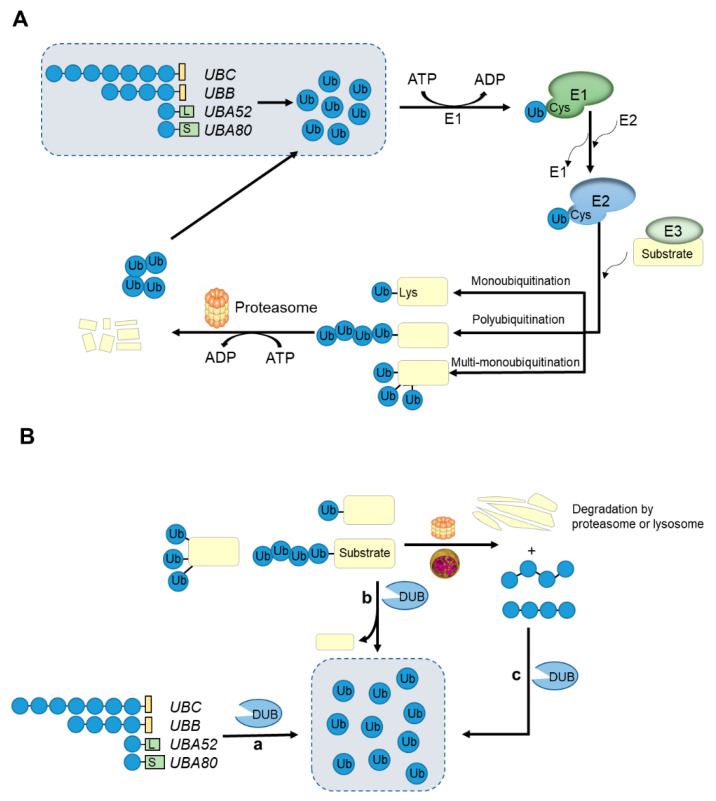
Figure 1.
An overview of the ubiquitin-proteasome system and the roles of deubiquitinases (DUBs). (A) Ubiquitin is conjugated to substrate proteins by the ATP-dependent successive actions of E1, E2, E3 enzymes that result in target proteins degrading in the 26S proteasome. Proteins can be modified by a single ubiquitin at one or multiple sites or by ubiquitin chains with different lengths and linked sites. (B) Ubiquitination is a reversible modification, which is performed by the deubiquitinases. Deubiquitinases (DUBs) can recycle ubiquitin in three ways to maintain the ubiquitin level in cells: (a) generate monomeric ubiquitin from precursor proteins encoded by four genes; (b) specifically remove ubiquitin or ubiquitin chains from substrate proteins; (c) recycle ubiquitin from proteins degraded by proteasomes or lysosomes.