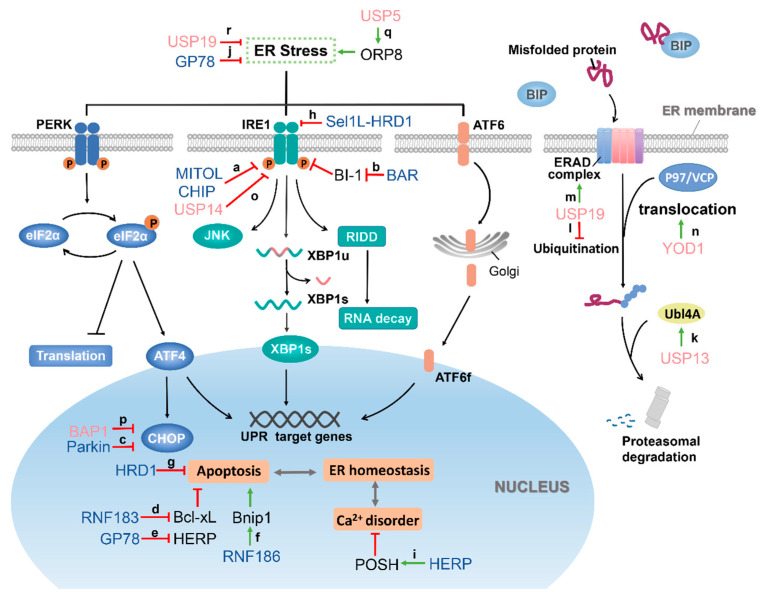
Figure 2.
The functional roles of E3 ubiquitin ligases (blue) and DUBs (pink) in the ER stress and ERAD signaling pathway. Once the unfolded protein is overloaded, the three signaling sensor proteins PERK (blue), IRE1 (green), and ATF6 (orange) that reside on the ER membrane are activated, and the activated PERK phosphorylates the eukaryotic translation initiation factor 2α (eIF2α), inhibiting global translation, reducing ER protein-load while also increasing transcription factor ATF4. Activated IRE1 triggers XBP1 mRNA splicing to initiate the synthesis of the transcription factor XBP1. It also enhances mRNA degradation, reduces the total protein content of the ER, and activates JNK and other cellular pathways. ATF6 is translocated into Golgi in response to ER stress and is cleaved to release the transcription factor ATF6f. These transcription factors, in turn, initiate a cascade of signals that relieve ER stress and restore homeostasis or induce cell death pathways. At the same time, the misfolded protein is reverse-transported to the cytoplasm with the assistance of the ERAD complex and the P97/VCP (valosin-containing protein) complex to complete the proteasome degradation. (a) The ubiquitination of IRE1α by mitochondrial ubiquitin ligase (MITOL) and carboxy-terminus of Hsc70-interacting protein (CHIP) inhibits its kinase activity, allowing for the suppression of ER stress. (b) BI-1 can interact with IRE1α and regulate its endonuclease activity. However, BI-1 is degraded by ubiquitination by bifunctional apoptosis regulator (BAR), leading to the release of the inhibitory effect of IRE1α, thereby promoting ER stress. (c) CHOP is violently induced when ER stress occurs, and overexpression of CHOP can lead to cell apoptosis. CHOP is ubiquitinated by Parkin to promote its degradation, which prevents ER stress-induced cell death. (d) Bcl-xL is an antiapoptotic protein and belongs to one of the Bcl-2 family members. RNF183 protein increases during ER stress occurs and ubiquitinates Bcl-xL to promote its degradation, leading to increased ER stress-induced apoptosis. (e) HERP (homocysteine-induced endoplasmic reticulum protein) is a negative regulator of ER stress, and it can protect cells from apoptosis mediated by ER stress. GP78 can interact with HERP, causing HERP to be ubiquitinated and degraded, thereby starting the process of ER stress recovery. (f) Bnip1 is a proapoptotic protein. RNF186 overexpression promotes Bnip1 ubiquitination and transfer to mitochondria but does not affect its protein level. Poly-ubiquitinated BNip1 amplifies RNF186-induced apoptosis signaling of ER stress. (g) HRD1 expression is upregulated by ER stress, and HRD1 rescues cells from ER stress-induced apoptosis. (h) Sel1L-HRD1 protein complex can recognize, ubiquitinate IRE1α, and mediate its degradation, restraining IRE1α signaling and reduces IRE1α-associated inflammation. (i) HERP also plays a vital role in maintaining calcium homeostasis during ER stress. POSH ubiquitinates HERP and promotes the redistribution of HERP from TGN to the ER, thereby regulating calcium homeostasis by increasing the level of HERP in the ER. (j) GP78 protects against ER stress in zebrafish liver, but the specific protective mechanism is not yet clear. (k) USP13 stabilizes UBL4A by deubiquitination, which is a functional component of ERAD, thus allowing proteasome degradation to proceed normally. (l) USP19 blocks the degradation of TCRa and other ERAD substrates through deubiquitination. (m) In addition, USP19 can deubiquitinate HRD1, which plays a key role in ERAD, and USP19 promotes ERAD by stabilizing HRD1. (n) YOD1 eliminates the Ub chains attached to substrates such as RI332 through deubiquitination and promotes the reverse translocation of substrates from the ER to the cytoplasm. (o) USP14 binds to IRE1 and inhibits its phosphorylation, thereby blocking IRE1-mediated cell death. (p) BAP1 directly binds to promoters of CHOP and inhibits the unfolded protein response (UPR) and associated apoptotic pathways by reducing its transcription. (q) ORP8 is a lipid transfer protein anchored to the ER, which can be deubiquitinated by USP5, causing disturbance of phospholipid transport and resulting in ER stress. (r) USP19 can inhibit the UPR and downregulate the expression of CHOP, but its specific target is unclear.