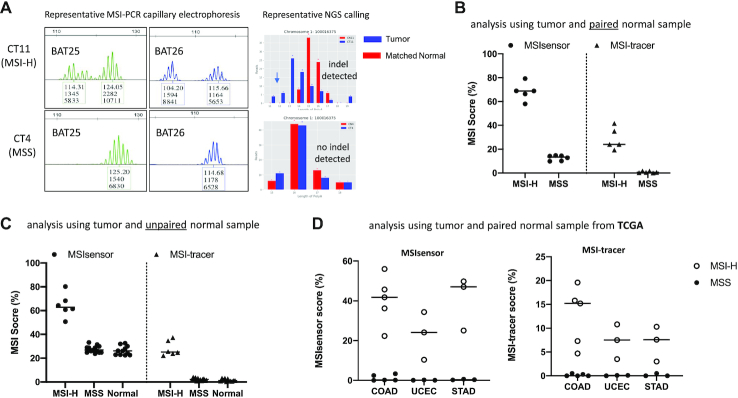
Figure 2.
MSI status analysis by inter-Alu-PCR using Microseq ultra-low pass sequencing (∼0.1–0.2 × 106 reads per sample). (A) Representative PCR-CE and NGS results from MSI-H and MSS tumor DNA. (B) Analysis of tumor and matched normal tissue samples. Inter-Alu-PCR was conducted on DNA from 11 colon cancer patients (five MSI-H and six MSS as assessed by the 5-plex PCR-CE assay). MSI status was evaluated via MSIsensor and MSI-tracer software. (C) Analysis of unpaired samples (tumor samples with un-matched normal tissue). Inter-Alu-PCR was applied on DNA from 18 tumors (5 MSI-H and 13 MSS) plus 11 normal tissues obtained from colon cancer patients, and were compared against human male control (HMC) DNA. (D) MSI analysis based on inter-Alu-PCR-obtained MS in different cancer types. Whole genome sequencing data for three MSI-prone cancer colon adenocarcinomas (COAD, n = 10), corpus endometrial carcinoma (UCEC, n = 6) and Stomach adenocarcinoma (STAD, n = 6) were obtained from TCGA database. Inter-Alu regions were extracted using bed files derived from inter-Alu-PCR Hiseq data. MSI status of MSS and MSI-H tumors were examined via MSIsensor and MSI-tracer.