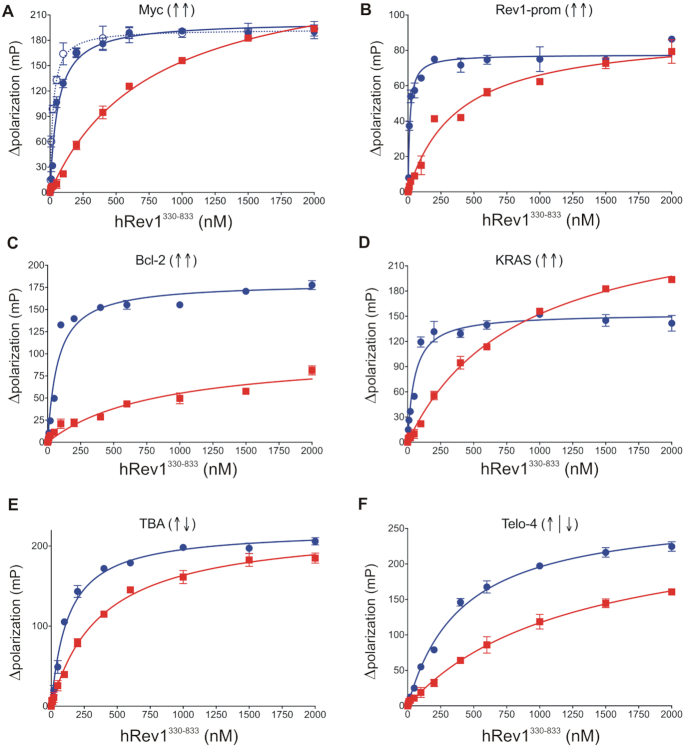
Figure 1.
hRev1330–833 preferentially binds to G4-forming DNA sequences, with a greater affinity for parallel-stranded G4 DNA than other G4 folds. The hRev1330–833 protein was titrated into a solution containing either single-stranded (ss)-G4-DNA (blue) or ss-non-G4-DNA (red) substrates at 1 nM. The range of concentrations for the protein is indicated on the X-axis. The change in fluorescence polarization at each concentration was measured and plotted as a function of the protein concentration. (A-F) Binding curves for hRev1330–833 core protein with the indicated G4 DNA substrate. In panel A, the binding curve for Myc-14/23 is shown as a solid blue line (full circles), while that for Myc-2/11 is shown as a dotted blue line (open circles). The G4 fold is indicated by the direction of arrows in parentheses for each panel (↑↑ = parallel G4, ↑↓ = anti-parallel G4, ↑|↓ = hybrid G4). Resulting data were fit to a quadratic equation to yield the binding dissociation constants given in Table 2. Reported values represent the mean ± SD (n = 3).