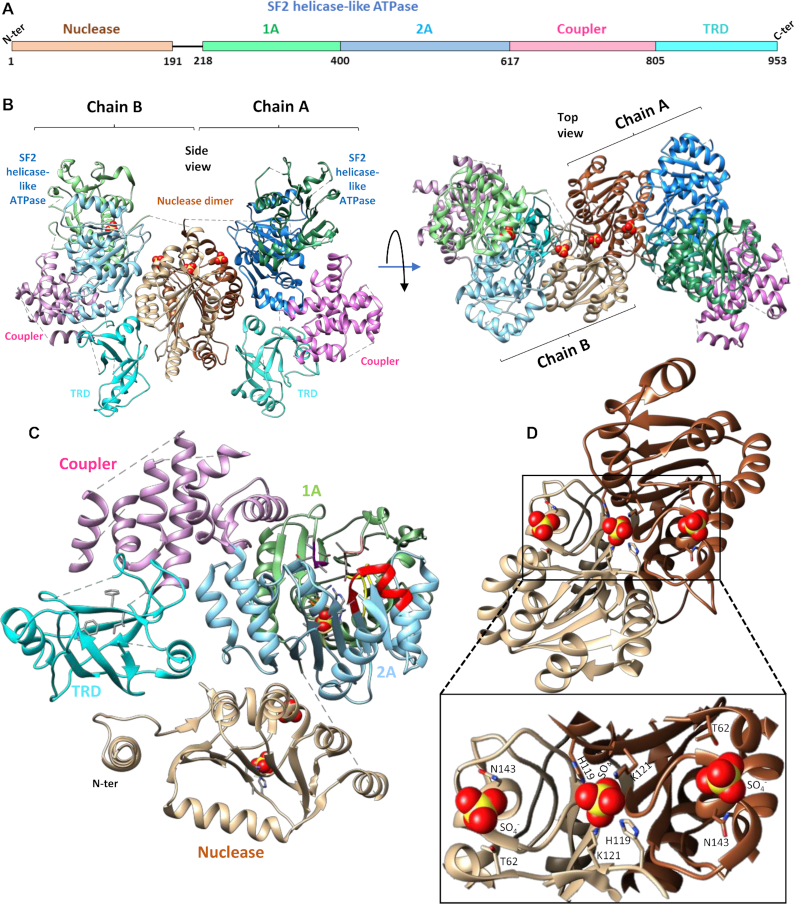
Figure 3.
Molecular architecture of SauUSI. (A) The primary domain arrangement in a SauUSI protomer, the line connecting the nuclease domain to the SF2 helicase-like ATPase signifies an unstructured linker. (B) Ribbon diagram of two views of the crystal structure of the dimeric SauUSI. Each structural domain of the protomers is colored distinctly. The sulfates are represented as spheres. (C) Structure of a protomer of SauUSI with nuclease domain in tan; the 1A and 2A domains of the ATPase having the RecA fold in green and blue, respectively; the coupler domain in pink; the TRD (SRA) domain in cyan. Certain important motifs/residues of domains are highlighted in sticks. (D) Structure of the nuclease from the two protomers highlighting the dimeric interface. A zoomed view of the DNA binding region of the nuclease dimer identified using the bound sulfate ions shown as spheres.