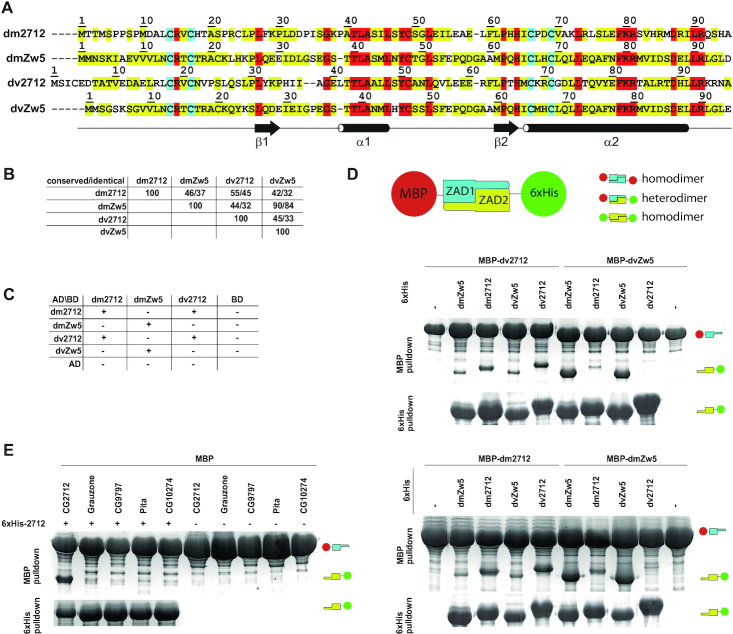
Figure 2.
Specificity of homodimerization retained between CG2712 and Zw5 orthologs. (A) Multiple sequence alignment of CG2712 and Zw5 zinc finger-associated domains (ZADs) from Drosophila melanogaster (dm) and D. virilis (dv). Identical residues are shown in red; conserved are in yellow, invariant cysteines are in cyan. (B) Percentage of conserved/identical residues in ZADs of CG2712 and Zw5 from D. melanogaster and D. virilis. (C) Testing of heterodimerization ability of dm/dv CG2712 and Zw5 ZADs in a yeast two-hybrid assay. The dvZw5 ZAD demonstrated strong self-activation properties when fused to Gal4 DNA-binding domain and was omitted for clarity. AD/BD denotes activation and DNA-binding domains of GAL4. (D) Testing of heterodimerization ability of dm/dv CG2712 and Zw5 ZADs in MBP or 6xHis-pulldown assay after co-expression in bacteria. The experiment scheme is shown on top. Small cartoons on the right show the positions of MBP-fused ZADs (MBP is 46kDa) and Thioredoxin-6xHis-fused ZADs (Thioredoxin-6xHis is 17kDa). On the scheme thioredoxin was not shown for clarity. Upper panels show results of interaction for MBP-fused D. virilis 2712 and Zw5 ZADs, bottom panels – for MBP-fused ZADs from D. melanogaster 2712 and Zw5. (E) Results of testing of heterodimerization ability between ZADs of CG2712 and proteins from other paralogous clusters in MBP or 6xHis-pulldown assay after co-expression in bacteria. Designations are as in panel D.