Figure 5.
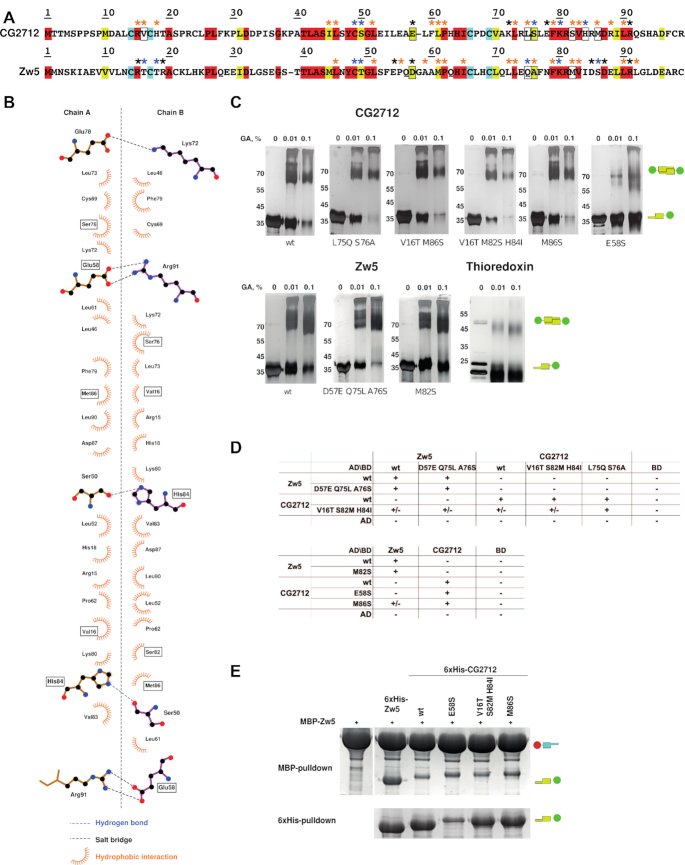
Site-directed mutagenesis suggested the cumulative effect of stabilizing interactions in the specificity of dimer formation. (A) Sequence alignment of CG2712 and Zw5 ZADs. Identical residues are shown in red, conserved – in yellow, invariant cysteines are in cyan. Blue asterisks show residues involved in hydrogen bonding, black – residues involved in salt bridges, while orange asterisks mark residues forming hydrophobic contacts according to molecular dynamics simulation (for further details see Supplementary Tables S5 and S6). Residues subjected to mutagenesis are shown in frame. (B) The plot of the CG2712 ZAD dimerization interface made for crystal structure. Point mutations introduced in CG2712 ZAD are shown in frame. (C) Results of testing the impact of substitutions on homodimerization using a chemical cross-linking assay with increasing concentrations of glutaraldehyde (concentrations are shown on the top). Thioredoxin was used as negative control. Positions of the molecular weight markers are shown on the left rows. Designations are as on Figure 2D. (D) Results of testing the effect of substitutions in a yeast two-hybrid assay. AD/BD denotes activation and DNA-binding domains of GAL4; +/- denotes weak growth. (E) Testing of the impact of point mutations on the heterodimerization ability of CG2712/Zw5 ZADs in a pulldown assay after co-expression in bacteria. Designations are as on Figure 2D. For complete results, see Supplementary Figure S6.