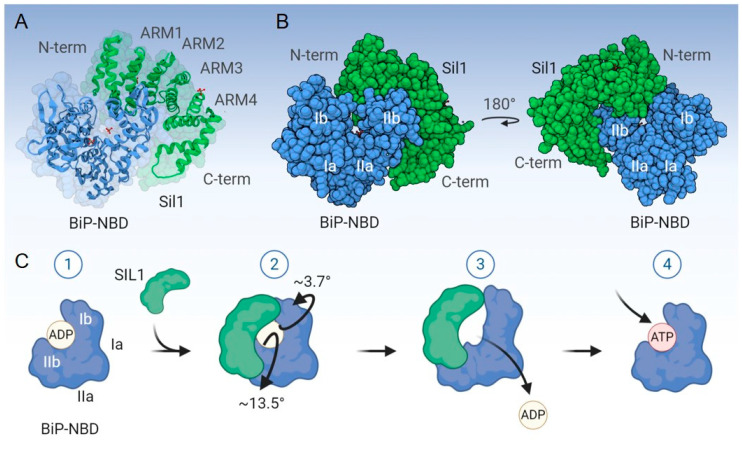
Figure 2.
Mechanism of nucleotide exchange by SIL1. (A,B) Displays the structure of a portion of S. cerevisiae Sil1 (Sil1p; green) complexed with the nucleotide-binding domain (NBD) of S. cerevisiae BiP (Kar2p; blue); PDB: 3QML. The N-terminus (N-term), C-terminus (C-term), and the four ARM domains of Sil1p are indicated, as are lobes Ia, Ib, IIa, and IIb of BiP’s NBD. (C) Demonstrates the mechanism of ADP to ATP exchange mediated by Sil1p. (1) Sil1p associates preferentially with the ADP-bound form of BiP. (2) Sil1p binds lobe IIb (major interaction site) as a “clamp” and also makes contact with lobe Ib (minor interaction site). The interaction of Sil1p with lobe Ib of BiP-NBD serves as the pivot point for Sil1p by which it introduces torsional strain in BiP’s NBD. (3) This, in turn, generates a conformational change in BiP’s NBD, swinging lobes IIb and Ib away from the nucleotide-binding pocket by ~13.5° and ~3.7°, respectively. These conformational changes abolish the hydrogen bonds between ADP and the respective residues from BiP’s NBD, which subsequently releases ADP from BiP and (4) enables BiP to enter a new cycle upon engaging its client substrate. Created with BioRender.com.