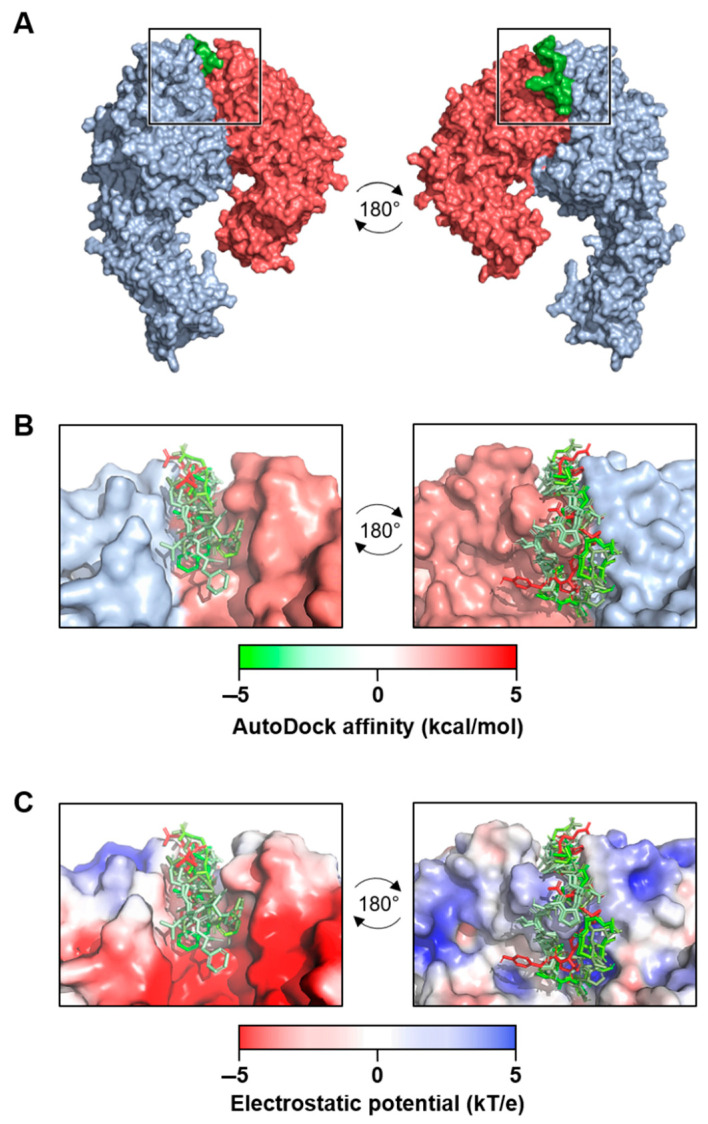
Figure 2.
Molecular docking analysis of extra domain A fibronectin (EDA-FN) peptide domain binding to the α4β1 receptor model. (A) The –TYSSPEDGHIEL-- peptide domain of the EDA-FN protein (green) was utilized as a ligand for molecular docking analysis to the integrin α4 (blue) β1 (red) receptor model. From the areas of the receptor analyzed, only the receptor binding cleft (boxed region) returned valid affinity score values. A 180° rotation is shown in order to visualize the peptide within the receptor binding cleft. (B) The top 7 binding modes of the EDA-FN peptide within the integrin receptor binding cleft. Green (–5) to red (+5) gradient colorization relates to the AutoDock affinity (kcal/mol) scores. (C) The electrostatic potential (kT/e) of the receptor binding cleft was predicted and is shown as a red (–5) to blue (+5) gradient. Note that the peptide had an overall stronger binding mode in the positive region of the binding cleft.