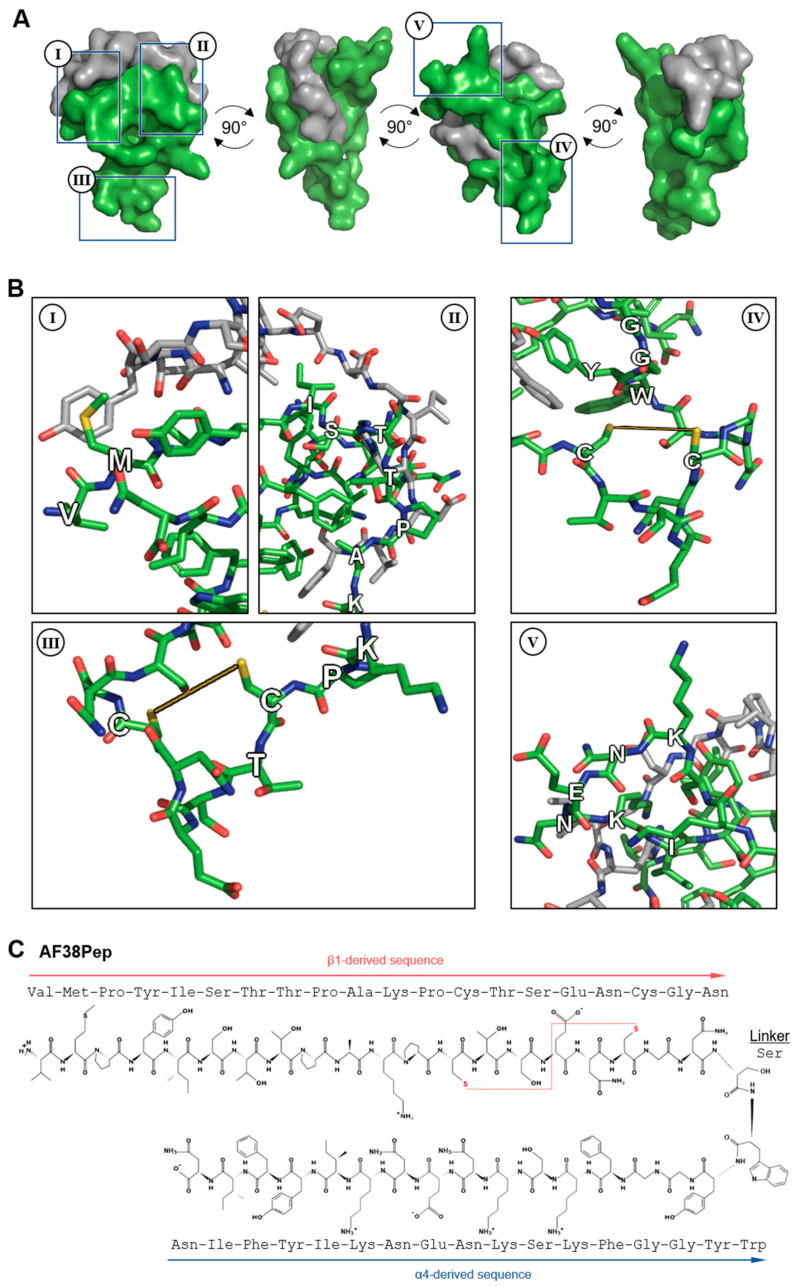
Figure 4.
Molecular docking analysis of the designed blocking polypeptide and key features. (A) The EDA-FN peptide (grey) was docked to the blocking polypeptide (green) derived from the polypeptide with the overall highest scores across all refinement analyses. A 360° rotation is shown to visualize the wrapping and binding of the EDA-FN peptide to the blocking polypeptide, which mimics the integrin α4β1/β7 receptor binding site. Boxed regions I–IV are shown in (B) to highlight the key features of the designed blocking polypeptide. I, the VM cap; II, the ISTTPAK motif of the β1/β7 subunit; III and IV, the C=C disulfide bridge to form the receptor and cage-like conformation; V, the KNENKI motif of the α4 subunit. (C) The antifibrotic 38-amino-acid polypeptide (AF38Pep) polypeptide sequence structure, showing two peptide chains derived from integrins β1 and α4, linked by a serine. Disulfide bridge is shown by a red dashed line, bridging the two cysteines.