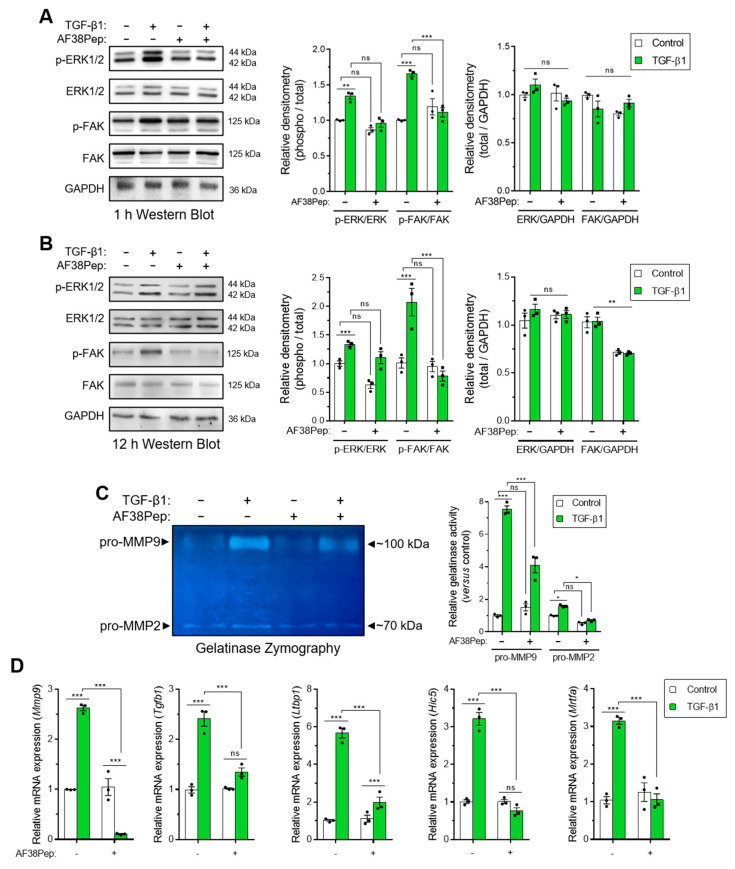
Figure 8.
Evaluation of blocking peptide capacity to inhibit integrin α4β1 signaling and downstream profibrotic effects. NIH/3T3 fibroblasts were incubated in the presence or absence of 10 ng/mL TGF-β1 with or without 10 µg/mL AF38Pep for (A) 1 h or (B) 12 h, before assessment of extracellular-signal-regulated kinases 1 and 2 (ERK1/2) and focal adhesion kinase (FAK) protein expression and phosphorylation. Immunoblots are displayed alongside corresponding densitometry analysis of phospho-protein/total-protein and total-protein/GAPDH loading control. (C) Gelatinase zymography indicating pro-matrix metalloproteinase 9 (pro-MMP9) (top band) and pro-MMP2 (bottom band) enzymatic activity following the indicated treatments for 48 h. Shown alongside is the quantification of relative gelatinase activity. (D) The mRNA expression for genes associated with integrin α4β1 activation: Mmp9, Tgfb1, Ltbp1, Hic5, and Mrtfa, which were assessed by real-time quantitative PCR (qRT-PCR) following 48 h of stimulation by 10 ng/mL TGF-β1. Blots, images, and data are representative of three independent experiments and data are shown as the mean ± S.E. Statistical analysis is shown as *** p ≤ 0.001, ** p ≤ 0.01, * p ≤ 0.05, and ns = no statistical significance (p > 0.05).