Abstract
Aim
Long non-coding RNA (lncRNA) SNHG17 has been shown to participate in type 2 diabetes mellitus, while its role in gestational diabetes mellitus (GDM) is unknown.
Methods
Quantitative real-time PCR (qRT-PCR) assays were conducted to compare the differential expression of SNHG17 among 60 GDM patients and 60 healthy pregnant female controls. In addition, peripheral blood samples from 240 pregnant females were collected to evaluate the predictive value of SNHG17 for GDM patients. All females were followed-up until delivery to record the occurrence of GDM and perinatal outcomes. GDM-free curves were plotted to compare the occurrence of GDM between high- and low- SNHG17 expression groups. The diagnostic value of plasma SNHG17 for GDM was analyzed by ROC curve analysis. Moreover, the cell counting kit (CCK-8) assay was performed to evaluate the impact of SNHG17 on cell viability of INS-1, and the level of insulin secretion was detected by enzyme linked immunosorbent assay (ELISA) after overexpression or knockdown of SNHG17.
Results
SNHG17 was downregulated in GDM patients compared to normal pregnant females. Low plasma expression levels of SNHG17 were closely correlated with the high incidence rate of GDM (GDM-free curve). Remarkably, plasma expression levels of SNHG17 at 4 weeks before the diagnosis of GDM (diagnosed by standard method) can be used to distinguish (ROC curve) GDM patients (diagnosed during follow-up) from normal pregnant females (GDM was not diagnosed during follow-up).
Conclusion
Plasma circulating SNHG17 is downregulated in GDM and has predictive values.
Keywords: gestational diabetes mellitus, lncRNA SNHG17, prediction
Introduction
Alterations of the production of hormones in the placenta during pregnancy can result in the accumulation of glucose in the blood. In certain cases, the pancreas may fail to produce enough insulin and gestational diabetes mellitus (GDM) could occur.1,2 The incidence of GDM varies across different regions. It is estimated that GDM affects less than 6% of pregnant women in Europe.3 However, in China, about 15% of females will experience GDM during their pregnancy.4 GDM occurring at early pregnancy stages may result in poor pregnancy outcomes, such as cesarean section, preterm delivery, pre-eclampsia and neonatal jaundice.5 Even worse, occurrence of GDM may also increase the risk of type 2 diabetes after delivery.6
In most cases, GDM is diagnosed at 24 to 28 weeks of gestation and early preventive interventions are not applicable.7 Therefore, predicting GDM at an early pregnancy stage is a big challenge in clinical practices.7,8 Certain biomarkers, such as maternal age and body mass index, have been identified to predict GDM. However, the accuracy and reliability are poor.7,8 Long non-coding RNAs (lncRNAs) are not involved in the synthesis of proteins but can regulate gene expression at multiple levels.9 It is well-established that the occurrence and development of GDM involve changes in the expression levels of a large number of lncRNAs.10 However, predictive values of these lncRNAs for GDM remain largely unknown. A recent study reported that lncRNA SNHG17 is downregulated in patients with type 2 diabetes compared to that in the healthy controls. Further correlation analysis revealed that the expression levels of SNHG17 were negatively associated with high-density lipoprotein cholesterol (HDL-C), suggesting that SNHG17 might be involved in the development of T2DM by regulating lipid metabolism and adipogenesis,11 which is also closely correlated with GDM.12 Based on these findings, this study was carried out to detect the expression levels of lncRNA SNHG17 in GDM patients and evaluate its predictive values.
Patients and Methods
Research Subjects
This study was approved by the Ethics Committee of Xinxiang Central Hospital and complied with the Declaration of Helsinki. Retrospective analysis was conducted to detect the plasma expression levels of SNHG17 in 60 GDM patients (24 to 28 weeks of gestation) and 60 healthy pregnant females (24 to 28 weeks of gestation). All these participants were enrolled at our institution between May 2018 and May 2019. To further determine the predictive value of SNHG17 for GDM, a prospective cohort study was designed. In this analysis, a total of 240 pregnant females (age range from 23 to 35 years, mean age 28.8 ± 3.4 years, 9 to 10 weeks of gestation) were enrolled at our institution between August 2018 and January 2019. Fasting peripheral blood was collected from all pregnant women at a 1 week interval, and the level of fasting blood glucose was monitored. In addition, the oral glucose tolerance test (OGTT) was carried out if the level of fasting blood glucose was abnormal. The diagnosis of GDM is made based on one of the following 3 criteria: 1) fasting plasma glucose ≥ 92 mg/dl; 2) plasma levels of glucose at 1 h post glucose load ≥ 180 mg/dl; and 3) plasma levels of glucose at 2 h post glucose load ≥ 153 mg/dl.13 All females were followed-up until delivery to record the occurrence of GDM and perinatal outcomes. No history of diabetes was observed in all participants. Patients complicated with other severe clinical disorders, such as malignancies, severe infections, heart diseases or metabolic diseases, were excluded from this study. All patients signed the informed consent.
Blood Extraction and Plasma Preparation
The 60 GDM patients and 60 healthy pregnant females were fasted for at least 12 h on the next date of admission (before therapy), followed by blood (5 mL) extraction. For the 240 pregnant females included in the follow-up study, fasting blood (5 mL) was extracted every week for a total of 24 weeks. All blood samples were mixed with EDTA and centrifuged at 2400 × g for 10 min to prepare plasma samples. Plasma samples were frozen in liquid nitrogen and stored at −80 °C before use.
RNA Preparation
Isolation of total RNAs from plasma samples was performed using Trizol reagent (Invitrogen). RNA samples were subjected to DNA removal by incubating with gDNA eraser (Takara) at 37 °C for 2 h. RNA integrity was checked using 5% urine-PAGE gel. RNA concentration was measured using the NanoDrop 2000 Spectrophotometer.
RT-qPCR Assay
The expression levels of SNHG17 in plasma were measured every 4 weeks (at 14, 18 and 22 weeks of gestation) to evaluate its predictive value for GDM patients. RNA samples with satisfactory quality were subjected to reverse transcription using the QuantiTect Reverse Transcription Kit (QIAGEN) to prepare cDNA samples under the following conditions: 25 °C for 10 min, 55 °C for 20 min and 85 °C for 10 min. To measure plasma expression levels of SNHG17, the SYBR Green PCR Kit (Takara Bio) was used to prepare qPCR reactions. All qPCR reactions were performed in triplicate. Expression levels of SNHG17 were normalized to endogenous control GAPDH using the 2−ΔΔCt method. The sample with the biggest ΔCt value was set to “1”, and all other samples were normalized to this sample. Primer sequences were: 5ʹ-TGCTTGTAAGGCAGGGTCTC-3ʹ (forward) and 5ʹ-ACAGCCACTGAAAGCATGTG-3ʹ (reverse) for SNHG17; and 5ʹ-CTGGGCTACACTGAGCACC-3ʹ (forward) and 5ʹ-AAGTGGTCGTTGAGGGCAATG-3ʹ (reverse) for GAPDH. PCR reaction conditions were 95 °C for 1 min, then 40 cycles of 95 °C for 10 s and 58 °C for 55 s.
Cell Culture and Transfection
The INS-1 cell line, which was derived from insulinoma cells of rats, was obtained from Bioleaf Biotech (Shanghai, China). Cells were cultured in RPMI 1640 (GIBCO, CA, USA) containing 10% (v/v) fetal bovine serum (FBS), 100 U/mL penicillin and 100 U/mL streptomycin (Solarbio) and incubated in a humidified atmosphere with 5% CO2 at 37 °C. INS-1 cells were seeded into 6-well plates at a density of 5 × 105 cells/well and cultured for 24 h. Subsequently, cell transfection was conducted using Lipofectamine 2000 following the manufacturer’s instructions. For the ectopic overexpression of SNHG17, its sequence was synthesized and cloned into the pcDNA3.1 vector (pcDNA3.1-SNHG17). On the other hand, SNHG17-shRNA plasmid (sh-SNHG17) was used to silence SNHG17. After 48 h, transfected cells were collected and transfection efficiency was detected by qPCR.
Cell Viability
The cell counting kit (CCK-8) assay was performed to evaluate the impact of SNHG17 on cell viability of INS-1 cells. In brief, cells were seeded into a 96-well plate with 5 × 105 cells/well. Next, 10 mL CCK-8 solution was added to each well at each specified time point (24 h, 46 h and 72 h after transfection). Cell viability was determined based on the absorbance at 450 nm of each sample.
Assessment of Insulin Secretion and Total Insulin Content
INS-1 cells were cultured in medium containing 3.3 mM or 16.7 mM glucose for 24 h. The supernatant was collected to detect the level of insulin secretion by enzyme linked immunosorbent assay (ELISA). Insulin secretion data were normalized to the total insulin content of the cells collected from each well using RIPA buffer (Beyotime) as previously described.14
Statistical Analysis
Data were expressed as mean ± standard error of the mean (SEM) values. The unpaired t-test was used to compare two groups. Based on the plasma expression levels of SNHG17 on the day of admission, the 240 pregnant females included in the follow-up study were divided into high and low SNHG17 level groups with the median expression level of SNHG17 as a cutoff value (n = 120). GDM-free curves were plotted and compared between two groups by log rank test. The chi-squared test was performed to analyze the correlations between plasma expression levels of SNHG17 and adverse perinatal outcomes. The diagnostic value of plasma SNHG17 for GDM was analyzed by ROC curve analysis, in which GDM patients were used as true positive cases and healthy pregnant females were used as true negative cases. p < 0.05 was considered as statistically significant.
Results
SNHG17 is Downregulated in GDM
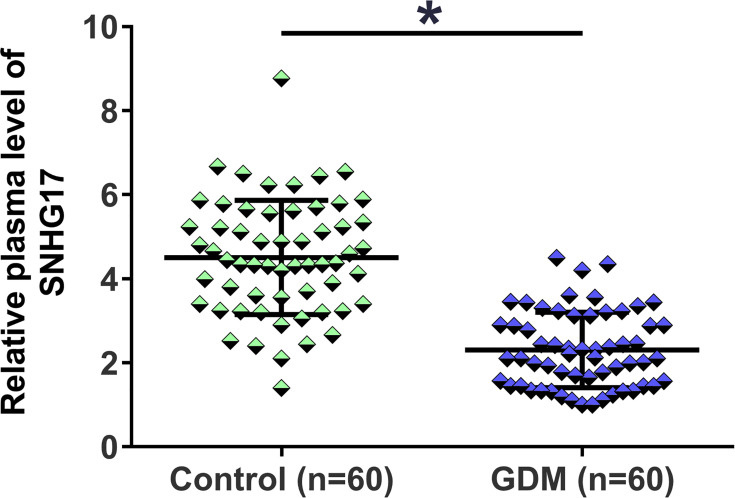
The characteristics of study subjects are shown in Table 1. There was no significant difference between GDM patients and healthy pregnant females in terms of age, pre-pregnancy BMI, delivery pattern and parity. The expression levels of SNHG17 in plasma from the 60 GDM patients and 60 healthy pregnant females (control) were measured by RT-qPCR. The relative expression levels of SNHG17 in the GBM group ranged from 1 to 4.23, and the relative expression levels of SNHG17 in the control group ranged from 1.27 to 8.89. Compared to the control group, significantly lower plasma expression levels of SNHG17 were observed in GDM patients (Figure 1, p < 0.05). These data suggested that SNHG17 was involved in GDM.
Table 1.
Baseline Characteristics of Study Subjects
| Variables | Study Subjects | p value | ||
|---|---|---|---|---|
| GDM (n = 60) | Control (n = 60) | |||
| Age (years) | 29.1 ± 3.1 | 28.6 ± 3.9 | 0.414 | |
| <32 | 53 (88.3%) | 56 (93.3%) | 0.343 | |
| ≥32 | 7 (11.7%) | 4 (6.7%) | ||
| Pre-pregnancy BMI (kg/m2) | 22.5 ± 2.7 | 21.8 ± 3.9 | 0.398 | |
| Fasting blood glucose (mmol/L) | 4.58 ± 0.43 | 5.01 ± 0.38 | <0.05 | |
| Delivery pattern | Vaginal | 32 (53.3%) | 37 (61.7%) | 0.356 |
| Cesarean | 28 (46.7%) | 23 (38.3%) | ||
| Parity | Primiparity | 36 (60.0%) | 39 (65.0%) | 0.572 |
| Multiparity | 24 (40.0%) | 21 (35.0%) | ||
Figure 1.
Comparison of plasma levels of SNHG17 expression in GDM patients and healthy pregnant females. The expression levels of SNHG17 in plasma from the 60 GDM patients and 60 healthy pregnant females were measured by RT-qPCR assays. All PCR reactions were repeated 3 times, and mean values were presented and compared. *p < 0.05.
Low Plasma Expression Levels of SNHG17 Predicted High Incidence Rate of GDM
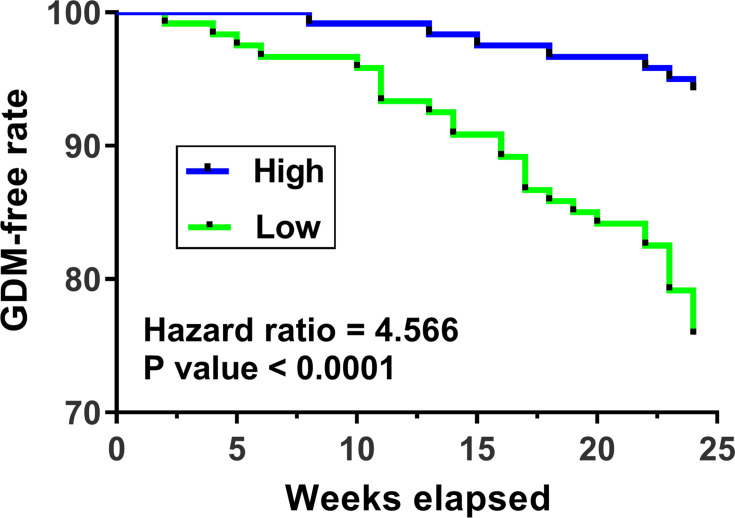
The 240 pregnant females (admitted at 2 months after pregnancy, blood glucose was within normal range on the day of admission) were followed up for 24 weeks. GDM-free curves were plotted and compared between the two groups. It was observed that patients in the low plasma SNHG17 level group showed a significantly higher incidence rate of GDM in comparison to patients in the high plasma SNHG17 level group (22% vs 3%; hazard ratio = 4.566, p < 0.0001; Figure 2). These data suggested that low plasma levels of SNHG17 might be used as a predictive biomarker for GDM.
Figure 2.
The relationship between the expression level of SNHG17 and the incidence of GDM. GDM-free curves were plotted and the incidence of GDM between patients with high and low SNHG17 expression was compared.
Plasma Expression Levels of SNHG17 at 22 Weeks of Gestation Have a Prediction Value for GDM
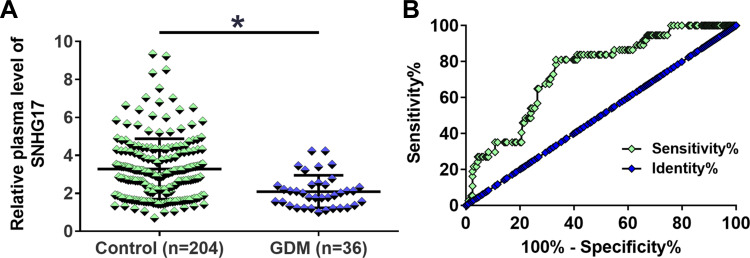
During the follow-up period, a total of 36 cases of GDM were diagnosed. It was observed that the plasma expression levels of SNHG17 at 22 weeks of gestation were significantly lower in 36 GDM patients in comparison to that in the 204 healthy pregnant females (p < 0.05; Figure 3A). With these 36 GDM patients as true positive cases and the rest of the healthy pregnant females (n = 204) as true negative cases, the diagnostic value of plasma expression levels of SNHG17 at each time point for GDM was analyzed by ROC curve analysis. Our results demonstrated that the plasma expression levels of SNHG17 at 22 weeks of gestation had a great predictive power for the diagnosis of GDM (cutoff value: 2.60, AUC: 0.744; 95% confidence interval: 0.666 to 0.823; sensitivity: 76.7%; specificity: 81.1%; p < 0.001; Figure 3B). In contrast, the expression levels of SNHG17 in plasma before this time point did not show a higher power to distinguish GDM patients from healthy pregnant females (AUC for at 14 weeks of gestation: 0.580; 95% confidence interval: 0.467 to 0.694; AUC for at 18 weeks of gestation: 0.650; 95% confidence interval: 0.564 to 0.736).
Figure 3.
The predictive value of SNHG17 expression level at 22 weeks of gestation for GDM. During the follow-up period, a total of 36 GDM patients were diagnosed. The plasma level of SNHG17 expression was measured and compared by RT-qPCR. It was observed that the plasma levels of SNHG17 at 22 weeks of gestation were significantly lower in 36 GDM patients than in 204 healthy pregnant females (A). *p < 0.05. The predictive value of SNHG17 expression for GDM was analyzed by ROC curve. The result showed that the plasma levels of SNHG17 expression at 22 weeks of gestation were sufficient to distinguish GDM patients from healthy pregnant females (B).
Correlations Between Plasma Levels of SNHG17 and Adverse Perinatal Outcomes
The chi-squared test was performed to analyze the correlations between plasma levels of SNHG17 and adverse perinatal outcomes. It was observed that plasma levels of SNHG17 were significantly correlated with preterm birth, but not low birth weight, admission to neonatal ICU and first minute birth asphyxia (Table 2).
Table 2.
Correlations Between Plasma Levels of SNHG17 and Adverse Perinatal Outcomes
| Items | Groups | Cases | High (n = 120) | Low (n = 120) | χ2 | p value |
|---|---|---|---|---|---|---|
| Stillbirth | Yes | 12 | 5 | 7 | 0.35 | 0.55 |
| No | 228 | 115 | 113 | |||
| Preterm birth | Yes | 25 | 7 | 18 | 5.40 | 0.02 |
| No | 215 | 113 | 102 | |||
| Low birth weight | Yes | 27 | 10 | 17 | 1.46 | 0.23 |
| No | 213 | 110 | 103 | |||
| Neonatal ICU | Yes | 12 | 7 | 5 | 0.35 | 0.55 |
| No | 218 | 113 | 115 | |||
| First minute birth asphyxia | Yes | 34 | 14 | 20 | 1.23 | 0.27 |
| No | 206 | 106 | 100 |
The Impact of SNHG17 on the Cellular Growth and Insulin Secretion of INS-1 Cells
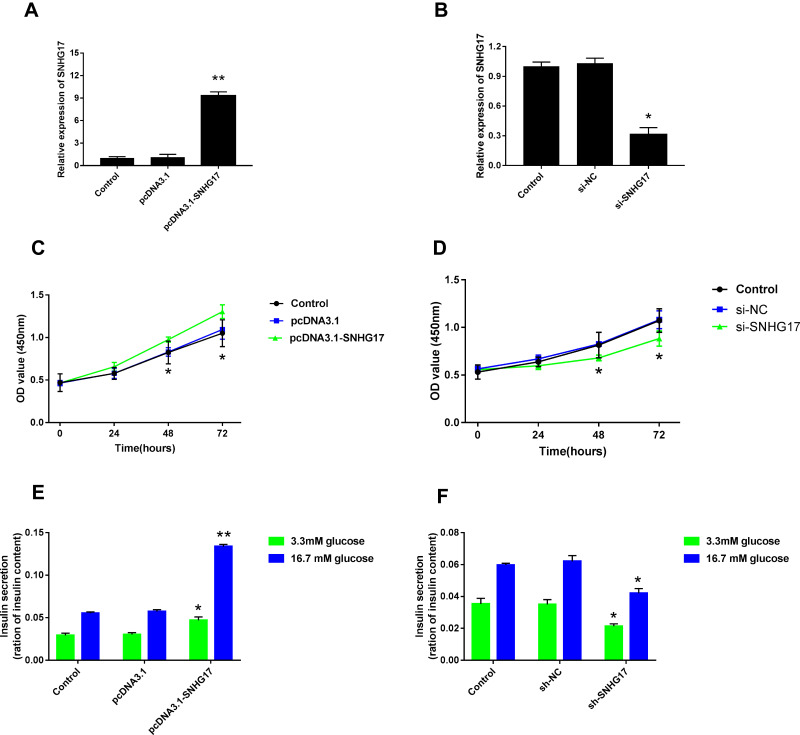
To further determine the role of SNHG17 in the development of GDM, INS-1 cells were transfected with pcDNA3.1-SNHG17 and sh-SNHG17 to overexpress and silence SNHG17, respectively. Compared with pcDNA3.1 vector transfection, the expression levels of SNHG17 were significantly elevated after pcDNA3.1-SNHG17 transfection (Figure 4A). Similarly, transfection of sh-SNHG17 markedly downregulated the expression of SNHG17 in INS-1 cells compared with the transfection of sh-normal control (NC) (Figure 4B). The CCK-8 assay indicated that overexpression of SNHG17 promoted the cell viability of INS-1 cells, while silencing of SNHG17 inhibited the growth of these cells (Figure 4C and D). In addition, overexpression of SNHG17 markedly increased insulin secretion of INS-1 cells, regardless of the glucose concentration (Figure 4E). By contrast, silencing of SNHG17 resulted in reduction of insulin secretion under both low (3.3 mM) and high (16.7 mM) glucose conditions (Figure 4F).
Figure 4.
The impact of SNHG17 expression on the cellular growth and insulin secretion of INS-1 cells. qPCR assay suggested that pcDNA3.1-SNHG17 transfection markedly elevated the expression level of lncRNA SNHG17 compared with pcDNA3.1 vector transfection (A). In addition, the expression level of SNHG17 was significantly downregulated by sh-SNHG17 transfection (B). The relationships between SNHG17 expression and cell viability of INS-1 cells were shown (C and D). Insulin secretions in low (3.3 mM) and high (16.7 mM) glucose conditions were assessed by ELISA assay after upregulation (E) and downregulation (F) of SNHG17 expression. *p < 0.05, **p < 0.01.
Discussion
This study investigated the involvement of SNHG17 in GDM. We found that SNHG17 is significantly downregulated in GDM. We also showed that downregulation of SNHG17 had early diagnostic value for GDM.
Differential expression of lncRNAs in GDM has been reported in previous studies.15–17 lncRNA MALAT1 is overexpressed in patients with GDM in comparison to that in healthy controls, and the upregulation of MALAT1 can be used to distinguish GDM patients from healthy controls.16 MEG3 is upregulated in human umbilical vein endothelial cells (HUVECs) from GDM patients and upregulation of MEG3 suppresses the migration, proliferation and angiogenesis but promotes the apoptosis of HUVECs.17 A recent study reported that downregulation of SNHG17 was closely correlated with the high prevalence of type 2 diabetes.11 To the best of our knowledge, our study is the first to report the downregulation of SNHG17 in GDM. We also revealed that downregulation of SNHG17 could inhibit the growth of insulin-producing cells and reduce the insulin secretion under both low and high glucose conditions. However, the mechanism and exact role of SNHG17 in the pathogenesis of GDM are still unknown. Whether SNHG17 participated in certain glucose uptake and metabolism pathways to impair the insulin secretion and release during GDM needs to be further investigated. In the future, molecular therapy targeting SNHG17 might become a novel and alternative treatment method for GDM.
Early application of preventive interventions may improve pregnancy outcomes.18,19 However, GDM only affects a small portion of pregnant females and it is not practical to apply preventive interventions for all pregnant women. Although previous studies have demonstrated that lncRNAs could be used to assist the diagnosis of GDM,16 biomarkers that can be used to predict GDM before its occurrence are still rare. An advantage of this study was that the blood sample was prospectively collected at the early-stage gestational period, highlighting the clinical significance of early biomarker detection. In this study, we showed that low plasma expression levels of SNHG17 predicted a high incidence rate of GDM. In addition, we also showed that plasma expression levels of SNHG17 at 22 weeks of gestation are sufficient to separate GDM patients from healthy pregnant females. Therefore, monitoring the changes in plasma expression levels of SNHG17 during pregnancy may help to identify individuals with high risk of GDM. Preventive interventions can be applied to these individuals to avoid the occurrence of GDM and/or improve pregnancy outcomes. However, whether SNHG17 could really improve or even replace the current diagnostic criteria for DMG remains to be further studied. The present study is limited by small sample size, and further studies are required to verify our findings. In addition, we also showed that low expression levels of SNHG17 were closely correlated with preterm birth. Future studies may focus on the prevention of preterm birth through the regulation of SNHG17.
Conclusion
The expression levels of SNHG17 in the plasma of GDM patients were significantly lower than those in healthy pregnant females, and might serve as a predictive biomarker for GDM.
Abbreviation
lncRNAs, long non-coding RNA; DNA, deoxyribonucleic acid.
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Landon MB, Gabbe SG. Gestational diabetes mellitus. Obstet Gynecol. 2011;118(6):1379–1393. doi: 10.1097/AOG.0b013e31823974e2 [DOI] [PubMed] [Google Scholar]
- 2.Ryan E. Diagnosing gestational diabetes. Diabetologia. 2011;54(3):480–486. doi: 10.1007/s00125-010-2005-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damm P, Houshmand-Oeregaard A, Kelstrup L, Lauenborg J, Mathiesen ER, Clausen TD. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. 2016;59(7):1396–1399. doi: 10.1007/s00125-016-3985-5 [DOI] [PubMed] [Google Scholar]
- 4.Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta‐analysis. J Diabetes Investig. 2019;10(1):154–162. doi: 10.1111/jdi.12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sweeting AN, Ross GP, Hyett J, et al. Gestational diabetes mellitus in early pregnancy: evidence for poor pregnancy outcomes despite treatment. Diabetes Care. 2016;39(1):75–81. doi: 10.2337/dc15-0433 [DOI] [PubMed] [Google Scholar]
- 6.Gunderson EP, Hurston SR, Ning X, et al. Lactation and progression to type 2 diabetes mellitus after gestational diabetes mellitus: a prospective cohort study. Ann Intern Med. 2015;163(12):889–898. doi: 10.7326/M15-0807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran TS, Hirst JE, Do MAT, Morris JM, Jeffery HE. Early prediction of gestational diabetes mellitus in Vietnam: clinical impact of currently recommended diagnostic criteria. Diabetes Care. 2013;36(3):618–624. doi: 10.2337/dc12-1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thériault S, Giguère Y, Massé J, Girouard J, Forest J-C. Early prediction of gestational diabetes: a practical model combining clinical and biochemical markers. Clin Chem Lab Med. 2016;54(3):509–518. doi: 10.1515/cclm-2015-0537 [DOI] [PubMed] [Google Scholar]
- 9.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47. doi: 10.1038/nrg.2015.10 [DOI] [PubMed] [Google Scholar]
- 10.Lu J, Wu J, Zhao Z, Wang J, Chen Z. Circulating lncRNA serve as fingerprint for gestational diabetes mellitus associated with risk of macrosomia. Cell Physiol Biochem. 2018;48(3):1012–1018. doi: 10.1159/000491969 [DOI] [PubMed] [Google Scholar]
- 11.Mohamadi M, Ghaedi H, Kazerouni F, et al. Deregulation of long noncoding RNA SNHG17 and TTC28-AS1 is associated with type 2 diabetes mellitus. Scand J Clin Lab Invest. 2019;79(7):519–523. doi: 10.1080/00365513.2019.1664760 [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Xu S, Chen H, Zhong L, Wang Z. The associations between triglyceride to high-density lipoprotein cholesterol ratios and the risks of gestational diabetes mellitus and large-for-gestational-age infant. Clin Endocrinol (Oxf). 2015;83(4):490–497. doi: 10.1111/cen.12742 [DOI] [PubMed] [Google Scholar]
- 13.Voormolen DN, Abell SK, James R, Hague WM, Mol BW. Diagnostic criteria and treatment for gestational diabetes mellitus. Semin Reprod Med. 2016;34(2):102–109. doi: 10.1055/s-0036-1572440 [DOI] [PubMed] [Google Scholar]
- 14.Jiao A, Li F, Zhang C, Lv W, Chen B, Zhang J. Simulated cholinergic reinnervation of β (INS-1) cells: antidiabetic utility of heterotypic pseudoislets containing β cell and cholinergic cell. Int J Endocrinol. 2018;2018:1–13. doi: 10.1155/2018/1505307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16(1):7. doi: 10.1007/s11892-015-0699-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Wu H, Wang F, Ye M, Zhu H, Bu S. Long non‐coding RNA MALAT 1 expression in patients with gestational diabetes mellitus. Int J Gynecol Obstet. 2018;140(2):164–169. doi: 10.1002/ijgo.12384 [DOI] [PubMed] [Google Scholar]
- 17.Ye H, Yang S, Zhang Y. MEG3 damages fetal endothelial function induced by gestational diabetes mellitus via AKT pathway. Eur Rev Med Pharmacol Sci. 2018;22(24):8553–8560. doi: 10.26355/eurrev_201812_16617 [DOI] [PubMed] [Google Scholar]
- 18.Donazar-Ezcurra M, López-del Burgo C, Bes-Rastrollo M. Primary prevention of gestational diabetes mellitus through nutritional factors: a systematic review. BMC Pregnancy Childbirth. 2017;17(1):30. doi: 10.1186/s12884-016-1205-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agha-Jaffar R, Oliver N, Johnston D, Robinson S. Gestational diabetes mellitus: does an effective prevention strategy exist? Nat Rev Endocrinol. 2016;12(9):533. doi: 10.1038/nrendo.2016.88 [DOI] [PubMed] [Google Scholar]