Abstract
Background:
Heart failure with preserved ejection fraction (HFpEF) is characterized by left ventricular hypertrophy (LVH) and reduced exercise capacity. Fibroblast growth factor 23 (FGF23), a hormone involved in phosphate, vitamin D and iron homeostasis, is linked to LVH and HF. We measured c-terminal FGF23 (cFGF23) and intact FGF23 (iFGF23) levels and examined their associations with exercise capacity in patients with HFpEF.
Methods:
Using multivariable linear regression and linear mixed models, we studied the associations of cFGF23 and iFGF23 with baseline and mean weekly change over 24 weeks in peak oxygen consumption (VO2) and 6-minute walk distance (6MWD) in individuals enrolled in the Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in HFpEF trial. Our study population comprised of 172 individuals with available plasma for cFGF23 and iFGF23 measurements.
Results:
Median (25th–75th percentile) baseline cFGF23 and iFGF23 levels were 208.7 (132.1–379.5) RU/ml and 90.3 (68.6–128.5) pg/ml, respectively. After adjustment for cardiovascular disease, hematologic and kidney parameters, higher cFGF23 was independently associated with lower peak VO2 at baseline. Higher iFGF23 was independently associated with shorter 6MWD at baseline. No significant associations were appreciated with the longitudinal outcomes.
Conclusion:
In patients with HFpEF, higher FGF23 levels are independently associated with reduced exercise capacity at baseline.
Keywords: Heart failure with preserved ejection fraction, fibroblast growth factor 23, exercise capacity, chronic kidney disease
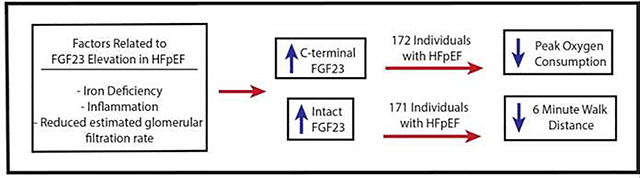
Graphical Abstract
Iron deficiency, inflammation, and reduced kidney function are potential factors that result in elevated c-terminal fibroblast growth factor 23 (cFGF23) and intact fibroblast growth factor 23 levels (iFGF23) in patients with heart failure with preserved ejection fraction (HFpEF). Both cFGF23 and iFGF23 were associated with at least one measure of reduced exercise capacity in patients with established HFpEF: cFGF23 was independently associated with reduced oxygen consumption and iFGF23 was associated with reduced 6 minute walk distance in patients with HFpEF.
Introduction
Heart failure with preserved ejection fraction (HFpEF) is an emerging public health epidemic with over 3.3 million adults diagnosed with HFpEF in the United States.(1) Of the total incident cases of heart failure, at least half are HFpEF.(1,2) HFpEF is characterized by elevation in left sided filling pressures, and is commonly manifested by concentric left ventricular remodeling or left ventricular hypertrophy on echocardiography and reduced exercise capacity.(3,4) Given that exercise intolerance is a cardinal manifestation of HFpEF that significantly contributes to morbidity, exercise capacity is commonly used as an endpoint for clinical trials in HFpEF.(5) Systemic and microvascular inflammation, endothelial dysfunction and alterations in myocardial bioenergetics are thought to contribute to exercise intolerance in patients with HFpEF; however, the exact pathophysiologic mechanisms of HFpEF remain under investigation.(6,7)
Fibroblast growth factor 23 (FGF23) is a bone-derived hormone involved in phosphate, vitamin D and iron homeostasis.(8) The biologically active intact FGF23 hormone has target effects in the kidney and parathyroid glands. Circulating FGF23 levels are determined by a balance between production of the full FGF23 protein and its post-translational modification through cleavage into C-terminal and N-terminal fragments within the osteocytes and bone marrow stromal cells.(8,9)Traditional regulators of FGF23 production include phosphate, calcium, parathyroid hormone and 1,25dihydroxyvitamin D. Other non-traditional regulators of FGF23 that increase FGF23 transcription include iron deficiency, inflammation and hypoxia.(9) Effects of these factors on FGF23 production and cleavage may be discerned by measuring FGF23 levels with two available assays. The c-terminal FGF23 (cFGF23) assay measures both the biologically active intact FGF23 (iFGF23) hormone and its C-terminal fragments, whereas the iFGF23 assay measures levels of only the intact FGF23 hormone.(8) In health, increased transcription due to iron deficiency and/or inflammation are matched to increased post-translational cleavage, resulting in elevated levels of circulating cFGF23, which measures both fragments and the intact hormone, but normal levels of iFGF23, which only measures the intact hormone.(8) In advanced chronic kidney disease (CKD), post-translational cleavage is impaired and increased transcription secondary to iron deficiency and/or inflammation result in elevation in both cFGF23 and iFGF23 levels.(8,10)
FGF23 is elevated in rare diseases of hypophosphatemia and in patients with CKD, in whom elevated FGF23 levels preserve normophosphatemia as kidney disease advances.(11) Chronic FGF23 elevation may be maladaptive. Observational data demonstrate significant associations between FGF23 and cardiovascular disease, specifically congestive heart failure, left ventricular hypertrophy, atrial fibrillation and hypertension, independent of kidney function.(12–15) Although most studies do not differentiate between heart failure subtypes, direct links between FGF23 and HFpEF pathogenesis are supported by mouse models that establish that FGF23 binding to fibroblast growth factor receptor 4 (FGFR4) on the myocardium results in cardiac myocyte hypertrophy.(16) Understanding the relationships between FGF23 and exercise impairment in patients with HFpEF may provide insights into the pathophysiology of HFpEF.
We performed an ancillary study in patients enrolled in the Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure with Preserved Ejection Fraction (RELAX) trial (17) to: 1) investigate the degree of cFGF23 and iFGF23 elevation in a HFpEF population with and without CKD, 2) study the relationship between FGF23 levels and known regulators of FGF23 homeostasis in the RELAX trial, and 3) investigate the associations of FGF23 with baseline, and 24-week exercise capacity in patients with HFpEF.
Methods
Study Population
The RELAX trial (Clinicaltrials.gov identifier: NCT00763867 2013) was a double-blind, placebo-controlled randomized clinical trial conducted by the Heart Failure Clinical Research Network to investigate whether phosphodiesterase-5 inhibition with sildenafil would improve exercise capacity in 216 individuals with diagnosed HFpEF.(17) Cardiopulmonary exercise testing (CPET) and 6-minute walk test (6MWT) were used to determine peak oxygen consumption and exercise capacity. For inclusion into the RELAX trial, individuals were required to have a normal ejection fraction (≥50%) and New York Heart Association (NYHA) class II through IV heart failure.(17) They were also required to have a peak oxygen consumption (peak VO2) of ≤ 60% of age- and sex- specific normal values and a respiratory exchange ratio of ≥ 1.0 on CPET screening. Additional requirements included either an N-terminal pro-brain-type natriuretic peptide (NT-pro BNP) ≥400 pg/mL or elevated left ventricular filling pressures on cardiac catheterization.(17) Individuals with an estimated glomerular filtration rate (eGFR) of < 20 ml/min/1.73m2 were excluded from the study.(17) All participants provided written and informed consent and the study was approved by institutional review boards of each of the participating sites.
In an ancillary study to the RELAX trial, we used stored samples to measure baseline plasma cFGF23 and iFGF23, and baseline serum hematologic markers associated with FGF23 regulation.(8) We aimed to investigate the degree of FGF23 elevation at baseline in an HFpEF population and its associations with baseline and 24-week oxygen consumption and exercise capacity. Our study population comprised of 172 individuals with samples available for baseline cFGF23 and iFGF23 measurement. CPET was available for 171 of these individuals, 6MWT was available for all 172 individuals at baseline. For our longitudinal analyses, 24-week CPET was available in 153 individuals and 24-week 6MWT was available in 152 individuals.
Primary Exposure
The primary exposures were plasma cFGF23 and iFGF23 concentrations. Frozen plasma samples were shipped from the University of Vermont Core Laboratory to the Immunoassay and Biomarker Laboratory at the Diabetes Research Institute at the University of Miami, where FGF23 measurements were performed. All samples had previously undergone a single thaw. Plasma cFGF23 and iFGF23 were measured by ELISA testing using kits from Immutopics, Inc. (San Clemente, CA). Estimates of intra-assay coefficients of variation (CV) were 3.9% and 4.5% for cFGF23 and iFGF23, respectively. The inter-assay CV was 4.2% for cFGF23 and 3.3% for iFGF23. Reference ranges for cFGF23 and iFGF23 are not standardized, but have been reported as 21–80 RU/ml for cFGF23 and 3.8–18.7 pg/ml for iFGF23 in elderly well-nourished adults >65 years of age with an eGFR ≥ 90 mL/min/1.73 m2.(18)
Outcomes
The primary outcomes were baseline peak VO2 measured by CPET and 6-minute walk test distance (6MWD). Both measurements have previously been described.(17) The CPET protocol in the RELAX trial utilized either cycle or treadmill ergometry. The protocol involved a 5-minute rest period where gas exchange data was collected, 3-minutes of low-level exercise and a symptom-limited incremental ramp of 10 watts per minute. Breath-by-breath data was captured using metabolic carts and securely transferred to the RELAX CPET Core Laboratory for VO2 measurements (Massachusetts General Hospital, Boston, MA). The highest 30 second median breath-by-breath VO2 during the final minute of incremental exercise was determined to be the peak VO2.(19) Longitudinal outcomes were 24-week peak VO2 and 6MWD.
Covariates
Information on participant demographics, medication use and clinical data including self-reported history of diabetes and smoking were collected at the baseline RELAX trial visit. Given the relationship between iron deficiency and FGF23,(9) and that iron deficiency is associated with poor outcomes in heart failure with reduced and preserved ejection fraction,(20,21) we measured iron parameters in the RELAX trial as potential confounders. Serum iron, ferritin, and iron binding capacity were measured on a Roche Cobas 6000 analyzer using manufacturer’s reagent and protocols for instrument set up, operation and analyte analyses (Roche Diagnostics, Indianapolis, IN) at the University of Miami using stored frozen serum. Iron deficiency was defined as ferritin <100 ng/mL or ferritin 100–299 ng/mL and TSAT < 20%. Transferrin saturation (TSAT) was calculated by the formula iron / (iron + iron binding capacity). Serum high-sensitivity C-reactive protein (CRP), phosphate, hemoglobin, and NT-proBNP were measured via standard assays. Chronotropic index was computed using baseline CPET data with the formula (peak exercise heart rate [HR] – resting HR) / (age-predicted maximal HR – resting HR), as previously done.(22) Estimated glomerular filtration rate (eGFR) was calculated using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.(23) CKD was defined as an eGFR ≤ 60 ml/min/1.73m2.
Statistical Analysis
We first investigated the degree of FGF23 elevation in the HFpEF population by CKD status using cFGF23 and iFGF23 quartiles. Spearman correlation coefficients were then estimated between cFGF23 and iFGF23 in the whole population, and with each of our two primary outcomes, peak VO2 and 6MWD. We used linear regression models to investigate predictors of cFGF23 and iFGF23. We used linear regression models to examine the associations between baseline plasma cFGF23 and iFGF23, expressed as continuous variables, and peak VO2 and 6MWD. We hierarchically adjusted for demographics (age, gender, race), cardiovascular disease risk factors (diabetes, systolic blood pressure, CRP, smoking status, body mass index, NT-proBNP, eGFR, chronotropic index). Given biologic relationships between hematologic parameters of iron deficiency and anemia with FGF23, in our final model, we adjusted for TSAT, ferritin and hemoglobin. CPET exercise modality, cycle or treadmill, was also included as a covariate in our models with peak VO2 as the primary outcome. cFGF23 and iFGF23 were log transformed for all analyses.
Given relationships between sex and exercise capacity,(24) we assessed whether the association of cFGF23 and iFGF23 with peak VO2 and 6MWD was modified by sex. We also tested if our associations were modified by eGFR and diabetes by testing the significance of the interaction terms of each with cFGF23 and iFGF23.
For longitudinal analyses that investigated the associations of baseline cFGF23 and iFGF23 with 24-week peak VO2 and 6MWD, we performed linear mixed models for repeated measures with an unstructured covariance structure using baseline, 12-week and 24-week measurements. Time was considered a fixed effect. We used hierarchical adjustment approach in the linear mixed models similar to our primary analyses and additionally adjusted the models for baseline measurements. Given the RELAX Trial was a randomized interventional trial, we also adjusted our final models for randomization arm.
All analyses were performed using SAS version 9.4 (Cary, NC, USA). Two-sided P values < 0.05 were considered statistically significant.
Results
Baseline Descriptive Characteristics
Baseline characteristics of the study population are presented in Table 1, and were similar to the overall RELAX trial participants.(17) The mean age of the study population was 68.4 ± 10.0 years, 50.6% were female and 3.5% were black. The mean eGFR in the overall study population was 63.9 ± 22.0 ml/min/1.73m2 and median CRP in the study population was 3.7 (1.7 – 8.5) mg/L. More than half of the study population (58.5%) had iron deficiency (Table 1). Baseline exercise capacity was reduced in our study population (mean peak VO2 12.3 ± 3.0 ml/min/kg, mean 6MWD 315.3 ± 105.4 m). Median baseline cFGF23 level was 208.7 (132.1 – 379.5) RU/ml and median baseline iFGF23 level was 90.3 (68.6 – 128.5) pg/ml.
Table 1.
Baseline Characteristics of the Total Study Population
| Baseline characteristics | Number | |
|---|---|---|
| Age, years | 172 | 68.4 ± 10.0 |
| Female, N (%) | 172 | 87 (50.6) |
| Black, N (%) | 172 | 6 (3.5) |
| Current Smoking, N (%) | 172 | 26 (15.1) |
| Hypertension, N (%) | 172 | 145 (84.3) |
| Diabetes, N (%) | 172 | 69 (40.1) |
| NYHA Functional Class | 172 | |
| NYHA Functional Class 2, N (%) | 85 (49.4) | |
| NYHA Functional Class 3, N (%) | 87 (50.6) | |
| >1 heart failure hospitalization in prior year, N (%) | 172 | 78 (45.4) |
| Ace/ARB use, N (%) | 172 | 114 (66.3) |
| BMI, kg/m2 | 172 | 33.9 ± 7.2 |
| SBP, mmHg | 172 | 127.2 ± 17.0 |
| eGFR, ml/min/1.73m2 | 170 | 63.9 ± 22.0 |
| High sensitivity CRP, mg/L | 172 | 3.7 (1.7 – 8.5) |
| Phosphate, mg/dL | 164 | 3.5 ± 0.6 |
| CPET Hemoglobin, mg/dl | 166 | 13.1 ± 1.5 |
| Core lab NT-proBNP, pg/ml | 171 | 648.1 (220.8 – 1388.0) |
| Intact FGF23, pg/ml | 172 | 90.3 (68.6 – 128.5) |
| C-terminal FGF23, RU/ml | 172 | 208.7 (132.1 – 379.5) |
| Iron, ug/dL | 164 | 79.8 ± 32.2 |
| Ferritin, ng/mL | 164 | 112.4 (58.3 – 209.8) |
| TSAT, % | 164 | 23.9 ± 10.5 |
| Iron Deficiency, N (%) | 164 | 96 (58.5) |
| Exercise Mode | 171 | |
| Exercise Mode, Treadmill % | 57 (33.3) | |
| Exercise Mode, Bicycle % | 114 (66.7) | |
| Chronotropic Index | 169 | 0.5 ± 0.3 |
| Ejection fraction, % | 171 | 60.9 ± 7.2 |
| Left ventricular mass, g | 127 | 171.6 ± 69.6 |
| Left ventricular end systolic volume, ml | 94 | 43.4 ± 20.2 |
| Left ventricular end diastolic volume, ml | 94 | 118.6 ± 34.4 |
Results are reported as means ± standard deviation, proportions or medians with interquartile ranges
Abbreviations: cFGF23, c-terminal fibroblast growth factor 23; N, number; NYHA, New York Heart Association; Ace/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker; BMI, body mass index; SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate; CRP, c-reactive protein; CPET, cardiopulmonary exercise testing; NT-proBNP, N-terminal pro-brain natriuretic peptide, TSAT, transferrin saturation
Correlates of baseline cFGF23 and iFGF23
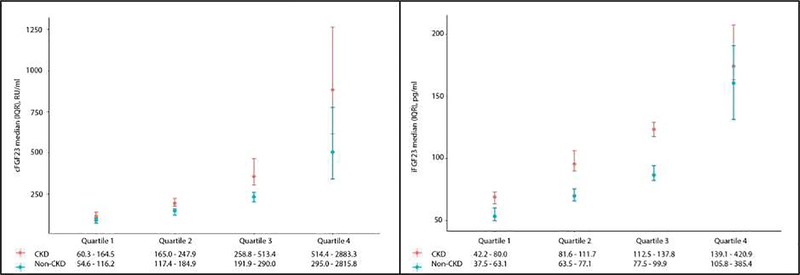
Within the HFpEF population with available eGFR data, 79 individuals had an eGFR value ≤ 60 ml/min/1.73m2 and were defined as having CKD. Ninety-one individuals did not meet criteria for CKD. Within the HFpEF population, cFGF23 and iFGF23 levels were higher in individuals with CKD than those without CKD (p values 0.003 and < 0.001, respectively). Median cFGF23 and iFGF23 levels were 247.9 (164.5, 513.4) RU/ml and 111.7 (80, 137.8) pg/ml in individuals with CKD and 184.9 (116.2, 290) RU/ml and 77 (63.1, 99.9) pg/ml in individuals without CKD. Both populations had higher FGF23 levels than previously reported normal ranges (Figure 1). cFGF23 was modestly correlated with iFGF23 (r = 0.32, p <0.0001). Systolic blood pressure, NT-pro-BNP and hematologic parameters of ferritin and hemoglobin were all significantly associated with cFGF23 in multivariable linear regression models (p value for all < 0.05). NT-pro-BNP was also significantly associated with iFGF23 (p value 0.04). Lower eGFR was independently associated with higher iFGF23 (p value 0.0001; Table 2), but the association between eGFR and cFGF23 was not significant in multivariable models.
Figure 1. cFGF23 and iFGF23 quartiles by CKD status in patients with HFpEF.
Quartiles of cFGF23 and iFGF23 in 79 individuals with CKD and 91 individuals without CKD. Results reported as medians with interquartile ranges. Abbreviations: cFGF23, c-terminal fibroblast growth factor 23; iFGF23, intact fibroblast growth factor 23; CKD, chronic kidney disease; HFpEF, heart failure with preserved ejection fraction; IQR, interquartile range
Table 2.
Determinants of cFGF23 and iFGF23 in multivariable linear regression models
| cFGF23 | iFGF23 | |||
|---|---|---|---|---|
| Covariates | F-value | p-value | F-value | p-value |
| Age | 1.26 | 0.26 | 3.68 | 0.06 |
| Female | 0.82 | 0.37 | 2.65 | 0.11 |
| Black | 0.05 | 0.83 | 1.39 | 0.24 |
| Diabetes | 0.09 | 0.76 | 0.18 | 0.67 |
| Systolic blood Pressure | 5.72 | 0.02 | 0.14 | 0.71 |
| C-reactive protein | 0.08 | 0.78 | 0.06 | 0.81 |
| Smoking | 0.53 | 0.47 | 0.27 | 0.60 |
| Body mass index | 0.01 | 0.93 | 0.01 | 0.90 |
| NT-proBNP | 22.2 | <.0001 | 4.44 | 0.04 |
| Chronotropic Index | 2.31 | 0.13 | 0.03 | 0.85 |
| eGFR | 0.02 | 0.90 | 15.77 | 0.0001 |
| Transferrin Saturation | 1.00 | 0.32 | 0.44 | 0.51 |
| Ferritin | 4.38 | 0.04 | 0.84 | 0.36 |
| Hemoglobin | 6.44 | 0.01 | 1.01 | 0.32 |
Model adjusted for age, gender, black race, diabetes, systolic blood pressure, C-reactive protein, smoking, body mass index, NT-proBNP, chronotropic index, estimated glomerular filtration rate, transferrin saturation, ferritin and hemoglobin
Abbreviations: NT-proBNP, N-terminal pro-brain natriuretic peptide; eGFR, estimated glomerular filtration rate
Associations of Baseline FGF23 and Baseline Exercise Capacity
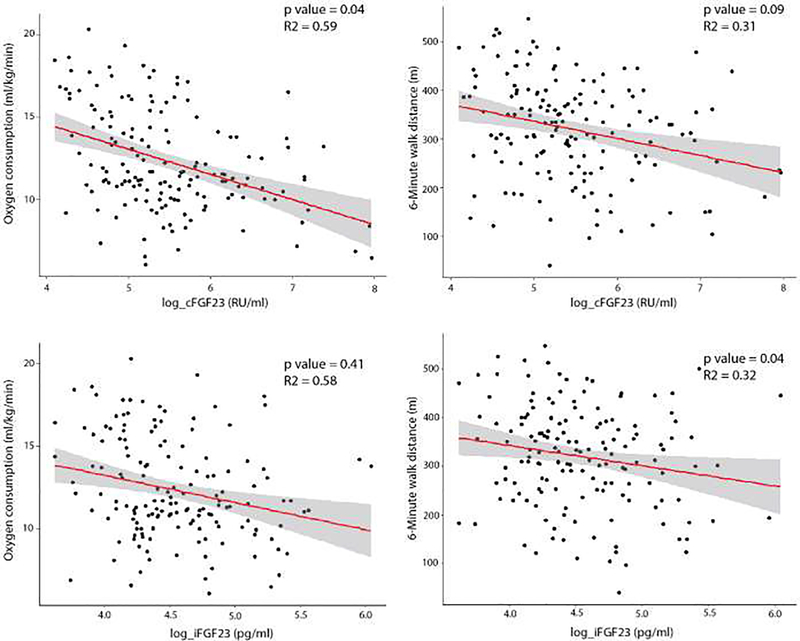
cFGF23 and iFGF23 were each negatively correlated with peak VO2 (cFGF23: r = − 0.37, p <0.0001; iFGF23: r = −0.26, p=0.0007) and 6MWD (cFGF23: r = −0.22, p=0.004; iFGF23: r = −0.18, p=0.02). In unadjusted linear regression models, higher cFGF23 was associated with lower peak VO2 (Table 3) and shorter 6MWD (Table 3). After adjusting for demographics (age, black race, sex, exercise modality), cardiovascular disease risk factors (diabetes, systolic blood pressure, CRP, smoking status, body mass index, NT-proBNP, eGFR, chronotropic index), and hematologic parameters (hemoglobin, TSAT, ferritin), cFGF23 remained significantly associated with lower peak VO2, but not with 6MWD (Table 3, Figure 2). In our fully adjusted multivariable linear regression models, iFGF23 was only significantly associated with 6MWD (Table 3, Figure 2).
Table 3.
Association of c-terminal FGF23 and intact FGF23 with baseline exercise capacity
| Baseline | Peak VO2 (ml/kg/min) | 6-Minute Walk Distance (meters) | ||||
| cFGF23 Models* | Parameter Estimate 95% CI | p - value | R2 | Parameter Estimate 95% CI | p - value | R2 |
| Unadjusted | −1.16 (−1.59 – −0.74) | <.001 | 0.15 | −22.79 (−38.37 – −7.22) | 0.004 | 0.05 |
| Model 1 | −1.08 (−1.46 – −0.70) | <.001 | 0.34 | −20.48 (−35.92 – −5.05) | 0.01 | 0.09 |
| Model 2 | −0.49 (−0.87 – −0.11) | 0.01 | 0.58 | −18.57 (−35.59 – −1.55) | 0.03 | 0.27 |
| Model 3 | −0.44 (−0.85 – −0.02) | 0.04 | 0.59 | −15.88 (−34.08 – 2.32) | 0.09 | 0.31 |
| iFGF23 Models* | Parameter Estimate 95% CI | p - value | Parameter Estimate 95% CI | p - value | ||
| Unadjusted | −0.75 (−1.19 – −0.31) | 0.001 | 0.06 | −20.66 (−36.31 – −5.02) | 0.01 | 0.04 |
| Model 1 | −0.63 (−1.03 – −0.23) | 0.002 | 0.26 | −19.29 (−34.86 – −3.71) | 0.02 | 0.09 |
| Model 2 | −0.19 (−0.55 – 0.18) | 0.31 | 0.56 | −18.52 (−34.68 – −2.37) | 0.02 | 0.27 |
| Model 3 | −0.16 (−0.54 – 0.22) | 0.41 | 0.58 | −17.22 (−33.73 – −0.71) | 0.04 | 0.32 |
Results presented as β-estimate per 1 SD increase in ln_cFGF23 and ln_iFGF23 in ml/kg/min for peak VO2 and meters for 6-minute walk distance
Model 1 adjusts for age, sex, black race, and exercise modality
Model 2 adjusts Model 1 variables plus diabetes, systolic blood pressure, C-reactive protein, smoking status, body mass index, NTpro-BNP, chronotropic index, and eGFR (CKD-EPI equation)
Model 3 adjusts for Model 2 plus transferrin saturation, ferritin, hemoglobin
Exercise modality was only included in the model when peak VO2 was the outcome
Abbreviations: FGF23, fibroblast growth factor 23; HFpEF, heart failure with preserved ejection fraction; CI, confidence intervals; NT-proBNP, N-terminal pro-brain natriuretic peptide; eGFR, estimated glomerular filtration rate
Figure 2. Associations of cFGF23 and iFGF23 with peak VO2 and 6MWD.*.
Scatter plot with regression line adjusted for age, sex, Black race, diabetes, exercise modality, systolic blood pressure, C-reactive protein, smoking status, body mass index, NTpro-BNP, chronotropic index, eGFR, transferrin saturation, ferritin, and hemoglobin. *No exercise modality in the model with 6MWD. Abbreviations: cFGF23, c-terminal fibroblast growth factor 23; iFGF23, intact fibroblast growth factor 23; 6MWD, 6-minute walk distance; NT-proBNP, N-terminal pro-brain natriuretic peptide; eGFR, estimated glomerular filtration rate.
The associations of higher cFGF23 and iFGF23 with peak VO2 or 6MWD were not modified by sex, eGFR, nor diabetes (P for interactions all > 0.18).
Associations of Baseline FGF23 and 24-Week Exercise Capacity
Among the153 individuals with 24-week VO2 data, mean (standard deviation) 24-week peak VO2 was 12.2 (3.2) ml/min/kg. Among the 152 individuals with 24-week 6MWD data, mean 24 week (standard deviation) 6MWD was 331.8 (108.0) meters. In fully adjusted linear mixed models that included randomization arm and baseline measures of exercise capacity, neither cFGF23 nor iFGF23 was associated with mean weekly change in exercise capacity over time (Table 4).
Table 4.
Association of c-terminal FGF23 and intact FGF23 with mean weekly change in exercise capacity over 24 weeks
| Mean weekly change in peak VO2 over 24 weeks (ml/kg/min) | P value | Mean weekly change in 6-Minute walk distance over 24 weeks (meters) | P value | |
|---|---|---|---|---|
| cFGF23 Models* | ||||
| Unadjusted | −0.084 (−0.200 – 0.031) | 0.08 | 0.19 (−4.32 – 4.69) | 0.86 |
| Model 1 | −0.083 (−0.199 – 0.033) | 0.08 | 0.22 (−4.28 – 4.73) | 0.88 |
| Model 2 | −0.086 (−0.203 – 0.032) | 0.07 | −0.09 (−4.66 – 4.48) | 0.73 |
| Model 3 | −0.069 (−0.191 – 0.053) | 0.17 | 0.97 (−3.70 – 5.64) | 0.83 |
| Model 4 | −0.071 (−0.192 – 0.051) | 0.16 | 1.40 (−3.37 – 6.16) | 0.63 |
| iFGF23 Models* | ||||
| Unadjusted | −0.135 (−0.289 – 0.018 | 0.05 | −2.40 (−8.51 – 3.70) | 0.25 |
| Model 1 | −0.133 (−0.286 – 0.021) | 0.05 | −2.34 (−8.45 – 3.76) | 0.26 |
| Model 2 | −0.137 (−0.293 – 0.019) | 0.04 | −2.50 (−8.70 – 3.70) | 0.24 |
| Model 3 | −0.128 (−0.290 – 0.033) | 0.07 | −2.32 (−8.59 – 3.95) | 0.26 |
| Model 4 | −0.125 (−0.287 – 0.037) | 0.08 | −2.23 (−8.65 – 4.19) | 0.30 |
Results presented as mean weekly change over 24 weeks in ml/kg/min for peak VO2 and meters for 6-minute walk distance with 95% confidence intervals
Model 1 adjusts for age, sex, black race, and exercise modality
Model 2 adjusts Model 1 variables plus diabetes, systolic blood pressure, C-reactive protein, smoking status, body mass index, NTpro-BNP, chronotropic index, and eGFR (CKD-EPI equation)
Model 3 adjusts for Model 2 plus transferrin saturation, ferritin, hemoglobin
Model 4 adjusts for Model 3 plus randomization arm and baseline exercise capacity
Exercise modality was only included in the model when peak VO2 was the outcome
Abbreviations: FGF23, fibroblast growth factor 23; HFpEF, heart failure with preserved ejection fraction; CI, confidence intervals; NT-proBNP, B-natriuretic peptide; eGFR, estimated glomerular filtration rate
Discussion
In participants enrolled in the RELAX trial, we performed an ancillary study to investigate the associations of baseline cFGF23 and iFGF23 with baseline and 24-week and oxygen consumption and 6MWD in patients with diagnosed HFpEF. Compared to previously reported normal reference ranges, levels of both cFGF23 and iFGF23 were elevated at baseline in patients with HFpEF with and without kidney disease enrolled in RELAX trial.(18) Higher levels of both cFGF23 and iFGF23 were associated with at least one marker of reduced exercise capacity. Higher baseline cFGF23 was independently associated with lower baseline peak VO2 in patients with HFpEF. Higher baseline iFGF23 was significantly associated with reduced 6MWD at baseline in patients with HFpEF. Neither was associated with longitudinal outcomes of exercise capacity.
Exact mechanisms of FGF23 elevation in HFpEF are unknown. Inflammation is one key regulator of FGF23 transcription.(9) Based on the American Heart Association and U.S. Centers for Disease Control and Prevention risk categories for high sensitivity CRP, the median CRP value in our patient population was categorized in the high risk group for cardiovascular disease.(25) The inflammatory milieu of HFpEF may represent one mechanism of increased cFGF23 levels in patients with HFpEF. Iron deficiency also upregulates FGF23 transcription,(9) and more than half of our study population had iron deficiency at baseline. Hematologic parameters were independently associated with cFGF23 at baseline, suggesting that iron deficiency may contribute to elevation in cFGF23 levels in patients with HFpEF. Kidney disease modifies FGF23 regulation, likely by increasing FGF23 transcription and reducing FGF23 cleavage.(26) Prior studies suggest that in advanced CKD, cFGF23 and iFGF23 are strongly correlated and most circulating FGF23 is the intact FGF23 hormone.(10) Our study supports this hypothesis as eGFR was the main determinant of iFGF23 in our study population. Underlying kidney dysfunction is one possible explanation for iFGF23 elevation in our patient population. Finally, it is also possible that cFGF23 and iFGF23 rise as a result of HFpEF pathology. Experimental data suggest a potential reverse causal relationship in which the altered myocardium is a source of circulating FGF23.(27) Together, increased production and reduced cleavage of FGF23 may establish HFpEF as a state of secondary cFGF23 and iFGF23 elevation, higher levels of which lead to or are a consequence of worse clinical parameters in HFpEF.
Studies investigating the associations of FGF23 with heart failure subtypes or in patients with HFpEF are limited. In the Multiethnic Studies of Atherosclerosis (MESA), iFGF23 was significantly associated with incident HFpEF.(28) iFGF23 was not associated with mortality in patients with existing HFpEF in the Ludwigshafen Risk and Cardiovascular Health (LURIC) Study.(29) In a recent study of 143 individuals with HFpEF, cFGF23 was significantly associated with cardiac fibrosis and mortality.(30) None of these studies included measurements of both cFGF23 and iFGF23.(28–30) A recent ancillary study to the Trial of Intensified verses Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF) measured both cFGF23 and iFGF23 and found levels of both were elevated, but neither was independently associated with mortality or heart failure hospitalizations.(31) Although TIME-CHF included individuals with preserved ejection fraction, the primary intent was not to study an HFpEF population and HFpEF constituted only one fifth of the total TIME-CHF study population.(31) Neither the MESA, LURIC nor TIME-CHF studies incorporated assessments of exercise capacity, an important endpoint in HFpEF. In our ancillary study that conducted simultaneous measurements of cFGF23 and iFGF23, we demonstrate that both cFGF23 and iFGF23 were associated with at least one measure of reduced exercise capacity at baseline in a population with established HFpEF.
There are multiple pathways that could explain the significant associations of FGF23 and exercise capacity. Circulating FGF23 may directly exert toxicity on cardiac myocytes through FGFR4 activation.(16) Blocking FGFR4 ameliorates this phenotype, suggesting a causal role for FGF23 in the pathogenesis of both heart failure with reduced and preserved ejection fraction.(32) FGF23 may activate the renin-angiotensin-aldosterone system via suppression of angiotensin converting enzyme-2, which may promote cardiac remodeling and fibrosis, features indicative of HFpEF.(33,34) FGF23 may indirectly contribute to HFpEF pathophysiology through inhibition of CYP27B1 and stimulation of CYP24A1 expression, resulting in decreased production and increased degradation of 1,25(OH)2D levels.(35) Low levels of vitamin D are associated with reduced functional capacity and poor clinical outcomes in patients with HFpEF.(36) FGF23 may also indirectly impact HFpEF through reduced bone marrow erythropoiesis and development of anemia, (37) which is associated with worse outcomes in patients with HFpEF.(22) FGF23 may also simply be a fidelity marker of more severe illness and inflammation. Most of these studies do not discriminate between the effects of cFGF23 and iFGF23 as investigators measured FGF23 levels with one assay. We find that both cFGF23 and iFGF23 were each associated with one marker of exercise capacity in patients with HFpEF. Regarding the discrepant results between our cross-sectional and longitudinal analyses, the absence of a substantial change in peak VO2 and 6MWD over the 24 week period may have limited our ability to detect significant associations.
Although we were able to investigate the cross-sectional and longitudinal associations between cFGF23 and iFGF23 and oxygen consumption and exercise capacity in a well-defined population with clear objective endpoints, we acknowledge additional limitations to our study. While significant associations were found between baseline FGF23 levels and baseline HFpEF parameters, cross-sectional analyses cannot prove causation. Our study is limited by single FGF23 measurements which also restricts our ability to make causal inferences. Although we have measurements of eGFR, we do not have measurements of proteinuria or 1,25(OH)2D in our study population. Finally, although we detected no significant interactions by sex, eGFR or diabetes status, the small sample size of our study did not allow us to conduct subgroup analyses or definitively exclude the possibility of effect modification by subgroups.
Whether FGF23 is a modifiable target that could result in improvements of cardiovascular disease is yet to be determined. Phosphate binders and dietary phosphate reduction may lower FGF23 levels in more advanced kidney disease,(38) but have not been studied in patients with HFpEF. Several studies demonstrate that treatment of iron deficiency can lower cFGF23 levels and improve functional status in heart failure with reduced ejection fraction, and may be one innovative approach to FGF23 lowering in HFpEF populations.(39,40) However, prior to implementation of FGF23 reducing strategies, further mechanistic studies investigating whether direct FGF23 toxicity contributes to HFpEF pathogenesis should be undertaken. Given the lack of guideline directed medical therapy for patients with HFpEF, identifying risk factors and pathophysiologic mechanisms of disease pathogenesis and severity remains an important area in need for further investigation.
Acknowledgments
Funding
This study was supported by grants P30DK114857, R01DK102438 (TI) R01 HL107577 (SJS), R01 HL127028 (SJS), R01 HL140731 (SJS), and R01 HL149423 (SJS), American Heart Association #16SFRN28780016 (SJS) and #19TPA34890060 (SSK), and a National Kidney Foundation of Illinois Young Investigator Grant (RM). Research reported in this publication was also supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number KL2TR001424 and by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. The Heart Failure Clinical Research Network is supported by the NHLBI, National Institutes of Health Funding/Support: U10 HL084904 (awarded to the coordinating center) and U10 HL110312, U10 HL110337, U10 HL110342, U10 HL110262, U10 HL110297, U10 HL110302, U10 HL110309, U10 HL110336, and U10 HL110338 (awarded to the regional clinical centers).
Conflict of Interest Statement
RM has interest in Abbott Laboratories, AbbVie, Inc. and Teva Pharmaceuticals Industries Ltd, and has received honoraria from Akebia/Otsuka. TI has received honoraria from Kyowa Hakko Kirin Co., Ltd and grant support from Shire. MW has received research support, honoraria or consultant fees from Amgen, Ardelyx, DiaSorin, Keryx, Lilly, Pfizer, Shire and Ultragenyx. GDL has received research support from Abbott, Actelion, Amgen, AstraZeneca, Cytokinetics, Corvia, Cyclerion, and Novartis; and has served as a consultant, scientific advisory board member, and/or executive committee/steering committee member for American Regent, Amgen, AstraZeneca, Cytokinetics, Cyclerion, Novartis, Pfizer and Sonivie. SJS has received research grants from Actelion, AstraZeneca, Corvia, Novartis, and Pfizer; and has served as a consultant, scientific advisory board member, and/or executive committee/steering committee member for Abbott, Actelion, AstraZeneca, Amgen, Axon Therapeutics, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Cardiora, CVRx, Cytokinetics, Eisai, GSK, Ionis, Ironwood, Merck, MyoKardia, Novartis, Pfizer, Sanofi, Shifamed, Tenax, and United Therapeutics.
Abbreviations
- 6MWD
6-minute walk distance
- 6MWT
6 minute walk test
- Ace/ARB
Angiotensin converting enzyme inhibitor/angiotensin receptor blocker
- BMI
Body mass index
- cFGF23
c-terminal FGF23
- CI
Confidence interval
- CKD
Chronic kidney disease
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- CPET
Cardiopulmonary exercise testing
- CRP
C-reactive protein
- C-terminal
Carboxy terminal
- CV
Coefficients of variation
- eGFR
Estimated glomerular filtration rate
- ELISA
Enzyme-linked immunosorbent assay
- FGF23
Fibroblast growth factor 23
- FGFR4
Fibroblast growth factor receptor 4
- HFpEF
Heart failure with preserved ejection fraction
- HR
Heart rate
- iFGF23
intact FGF23
- IQR
interquartile range
- LURIC
Ludwigshafen Risk and Cardiovascular Health
- MESA
Multiethnic Studies of Atherosclerosis
- N-terminal
Amino terminal
- NT-pro BNP
N-terminal pro-brain-type natriuretic peptide
- NYHA
New York Heart Association
- RELAX
Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure with Preserved Ejection Fraction
- RU
Relative units
- SAS
Statistical analysis software
- SBP
Systolic blood pressure
- SD
Standard deviation
- TIME-CHF
Trial of Intensified verses Standard Medical Therapy in Elderly Patients with Congestive Heart Failure
- TSAT
Transferrin saturation
- Peak VO2
Peak oxygen consumption
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Muntner P, Alonso A et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Tsao CW, Lyass A, Enserro D et al. Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC Heart Fail 2018;6:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redfield MM. Heart Failure with Preserved Ejection Fraction. The New England journal of medicine 2017;376:897. [DOI] [PubMed] [Google Scholar]
- 4.Smith JR, Borlaug BA, Olson TP. Exercise Ventilatory Efficiency in Older and Younger Heart Failure Patients With Preserved Ejection Fraction. Journal of Cardiac Failure 2019;25:278–285. [DOI] [PubMed] [Google Scholar]
- 5.Swank AM, Horton J, Fleg JL et al. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circulation Heart failure 2012;5:579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gevaert AB, Shakeri H, Leloup AJ et al. Endothelial Senescence Contributes to Heart Failure With Preserved Ejection Fraction in an Aging Mouse Model. Circulation Heart failure 2017;10. [DOI] [PubMed] [Google Scholar]
- 7.AbouEzzeddine OF, Kemp BJ, Borlaug BA et al. Myocardial Energetics in Heart Failure With Preserved Ejection Fraction. Circulation Heart failure 2019;12:e006240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf M, White KE. Coupling fibroblast growth factor 23 production and cleavage: iron deficiency, rickets, and kidney disease. Current opinion in nephrology and hypertension 2014;23:411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David V, Martin A, Isakova T et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney international 2016;89:135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimada T, Urakawa I, Isakova T et al. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. The Journal of clinical endocrinology and metabolism 2010;95:578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isakova T, Wahl P, Vargas GS et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney international 2011;79:1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scialla JJ, Xie H, Rahman M et al. Fibroblast growth factor-23 and cardiovascular events in CKD. Journal of the American Society of Nephrology : JASN 2014;25:349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta R, Cai X, Lee J et al. Association of Fibroblast Growth Factor 23 With Atrial Fibrillation in Chronic Kidney Disease, From the Chronic Renal Insufficiency Cohort Study. JAMA cardiology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutierrez OM, Januzzi JL, Isakova T et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009;119:2545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akhabue E, Montag S, Reis JP et al. FGF23 (Fibroblast Growth Factor-23) and Incident Hypertension in Young and Middle-Aged Adults: The CARDIA Study (Coronary Artery Risk Development in Young Adults). Hypertension 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grabner A, Amaral A, Schramm K et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metabolism 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redfield MM, Chen HH, Borlaug BA et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. Jama 2013;309:1268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chudek J, Kocelak P, Owczarek A et al. Fibroblast growth factor 23 (FGF23) and early chronic kidney disease in the elderly. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2014;29:1757–63. [DOI] [PubMed] [Google Scholar]
- 19.Redfield MM, Borlaug BA, Lewis GD et al. PhosphdiesteRasE-5 Inhibition to Improve CLinical Status and EXercise Capacity in Diastolic Heart Failure (RELAX) trial: rationale and design. Circ Heart Fail 2012;5:653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bekfani T, Pellicori P, Morris D et al. Iron deficiency in patients with heart failure with preserved ejection fraction and its association with reduced exercise capacity, muscle strength and quality of life. Clinical research in cardiology : official journal of the German Cardiac Society 2019;108:203–211. [DOI] [PubMed] [Google Scholar]
- 21.von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron Deficiency in Heart Failure: An Overview. JACC Heart failure 2019;7:36–46. [DOI] [PubMed] [Google Scholar]
- 22.Patel RB, Mehta R, Redfield MM et al. Renal Dysfunction in Heart Failure With Preserved Ejection Fraction: Insights From the RELAX Trial. J Card Fail 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honigberg MC, Lau ES, Jones AD et al. Sex Differences in Exercise Capacity and Quality of Life in Heart Failure With Preserved Ejection Fraction: A Secondary Analysis of the RELAX and NEAT-HFpEF Trials. J Card Fail 2020;26:276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson TA, Mensah GA, Alexander RW et al. Markers of Inflammation and Cardiovascular Disease. Circulation 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 26.Smith ER, Cai MM, McMahon LP, Holt SG. Biological variability of plasma intact and C-terminal FGF23 measurements. The Journal of clinical endocrinology and metabolism 2012;97:3357–65. [DOI] [PubMed] [Google Scholar]
- 27.Matsui I, Oka T, Kusunoki Y et al. Cardiac hypertrophy elevates serum levels of fibroblast growth factor 23. Kidney international 2018;94:60–71. [DOI] [PubMed] [Google Scholar]
- 28.Almahmoud MF, Soliman EZ, Bertoni AG et al. Fibroblast Growth Factor-23 and Heart Failure With Reduced Versus Preserved Ejection Fraction: MESA. Journal of the American Heart Association 2018;7:e008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandenburg VM, Kleber ME, Vervloet MG et al. Fibroblast growth factor 23 (FGF23) and mortality: the Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis 2014;237:53–9. [DOI] [PubMed] [Google Scholar]
- 30.Roy C, Lejeune S, Slimani A et al. Fibroblast growth factor 23: a biomarker of fibrosis and prognosis in heart failure with preserved ejection fraction. ESC Heart Fail 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stohr R, Brandenburg VM, Heine GH et al. Limited role for fibroblast growth factor 23 in assessing prognosis in heart failure patients: data from the TIME-CHF trial. European journal of heart failure 2020. [DOI] [PubMed] [Google Scholar]
- 32.Di Marco GS, Reuter S, Kentrup D et al. Treatment of established left ventricular hypertrophy with fibroblast growth factor receptor blockade in an animal model of CKD. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2014;29:2028–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia G, Aroor AR, Hill MA, Sowers JR. Role of Renin-Angiotensin-Aldosterone System Activation in Promoting Cardiovascular Fibrosis and Stiffness. Hypertension 2018;72:537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bockmann I, Lischka J, Richter B et al. FGF23-Mediated Activation of Local RAAS Promotes Cardiac Hypertrophy and Fibrosis. Int J Mol Sci 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutierrez O, Isakova T, Rhee E et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. Journal of the American Society of Nephrology : JASN 2005;16:2205–15. [DOI] [PubMed] [Google Scholar]
- 36.Nolte K, Herrmann-Lingen C, Platschek L et al. Vitamin D deficiency in patients with diastolic dysfunction or heart failure with preserved ejection fraction. ESC Heart Fail 2019;6:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta R, Cai X, Hodakowski A et al. Fibroblast Growth Factor 23 and Anemia in the Chronic Renal Insufficiency Cohort Study. Clinical journal of the American Society of Nephrology : CJASN 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehta R, Isakova T. Continued Search for Therapies to Favorably Modify Phosphate and FGF23 Levels in CKD. Clin J Am Soc Nephro 2017;12:1911–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2013;28:1793–803. [DOI] [PubMed] [Google Scholar]
- 40.Anker SD, Comin Colet J, Filippatos G et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. The New England journal of medicine 2009;361:2436–48. [DOI] [PubMed] [Google Scholar]