Figure 4. The PD-L1:B7–1 cis-interaction offers new mechanistic insight into CTLA-4-Ig therapy.
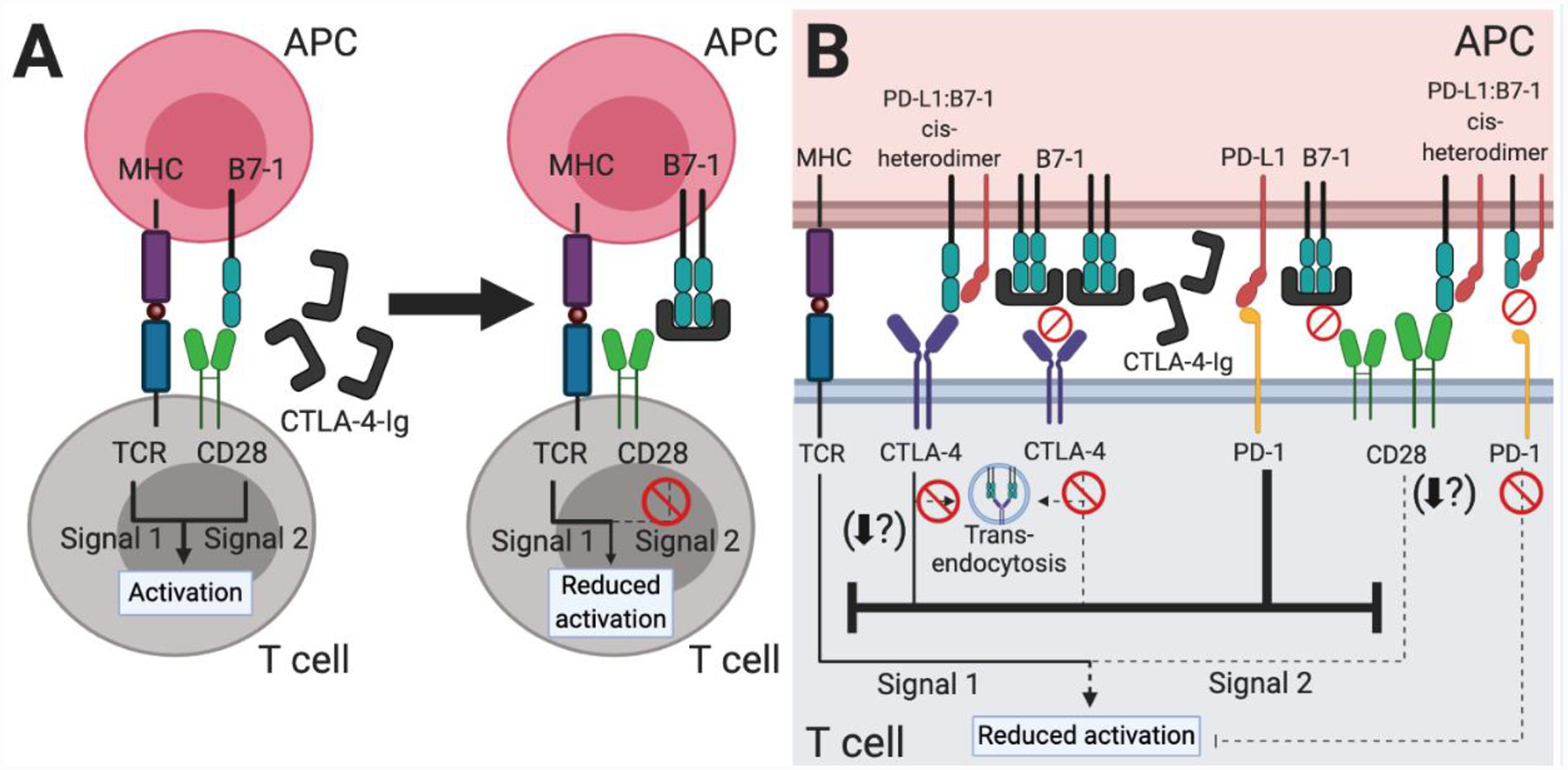
(A) Based on our original understanding of immune checkpoint signaling pathways (Figure 1A), CTLA-4-Ig therapy functions by decreasing CD28-mediated T cell costimulation. The fusion protein mimics the native CTLA-4 protein and binds to B7–1 and B7–2 (not shown) on APCs, thus preventing a CD28:B7–1 or B7–2 interaction. (B) Our new understanding of the PD-L1:B7–1 cis-interaction shows additional immune checkpoint signaling pathways which underlie CTLA-4-Ig therapy. As CTLA-4-Ig sequesters most free B7–1 and B7–2 (not shown), the B7–1 receptors CD28 and CTLA-4 do not transduce activating or inhibitory signals, respectively. PD-L1 is unaffected and may continue to bind PD-1, inhibiting signals one and two of T cell activation. PD-L2 (not shown) may also continue to inhibit T cell activation. Although likely sparse, PD-L1:B7–1 cis-heterodimers may continue to modulate CTLA-4, CD28, and PD-1 signaling. The PD-L1:B7–1:CTLA-4 interaction reduces B7–1 trans-endocytosis and potentially other CTLA-4 functional outcomes as well. Most studies indicate that it mediates continued CD28 costimulation, although one study demonstrated that it reduced T cell activation. As the PD-L1:B7–1 cis-heterodimer cannot bind PD-1, it does not further inhibit T cell activation through this pathway. Taken together, this therapy is highly immunosuppressive, but the preformed PD-L1:B7–1 cis-heterodimer may continue to interact with CD28 and provide enough activation over the long-term to mediate low levels of T cell responses.
