Abstract
Rationale:
Animal models of compulsive drug use that continues despite negative consequences can be used to investigate the neural mechanisms of addiction. However, models of punished or aversion-resistant opioid self-administration are notably lacking.
Objectives:
We sought to develop an aversion-resistant, oral fentanyl self-administration paradigm.
Methods:
In Experiment 1, C57BL/6J male and female, adult mice consumed fentanyl (10 μg/mL) in a two-bottle, drinking in the dark task and escalating concentrations of quinine were added to the bottles. In Experiment 2, mice were trained to administer oral fentanyl (10 μg/mL) in an operant response task. Quinine was next added to the fentanyl solution in escalating concentrations. In Experiment 3, mice were trained to respond for oral fentanyl or fentanyl adulterated with 500 μM quinine on every session. In Experiment 4, mice were trained to respond for a 1% sucrose solution before introduction of quinine.
Results:
Quinine reduced two-bottle choice consumption in males but not in females. Both sexes demonstrated the ability to detect the selected concentrations of quinine in fentanyl. In the operant chamber, mice responded robustly for oral fentanyl but introduction of quinine at any stage of training was insufficient to reduce responding. In contrast, quinine reduced responding for sucrose at concentrations above 250 μM.
Conclusions:
Mice will respond for and consume oral fentanyl in both a two-bottle choice and an operant-response task. Quinine is detectable in fentanyl but mice will continue to respond for and consume fentanyl with quinine in both paradigms. These data support the use of these models in behavioral studies of compulsive-like opioid use.
Keywords: addiction, opioid, mouse, compulsive, quinine
Introduction
Drug addiction is characterized by uncontrollable drug use that persists in the face of serious negative consequences (i.e., compulsiveness) (Everitt and Robbins, 2005). This presents a major challenge to those suffering from addiction and is thought to involve aberrant activity in brain reward systems (Kenny, 2007). Despite the fact that addiction is a significant problem that affects millions of individuals worldwide, treatments are limited in both the options available as well as their effectiveness (Veilleux et al., 2010; Peacock et al., 2018). As such, preclinical animal research investigating the neural mechanisms behind this problem is critically needed to advance treatment options and to understand the biology of this disease.
In the United States, rates of illicit opioid use and overdose have skyrocketed in recent years. This problem has overwhelmed communities and presents a major public health challenge. In 2017, 67.8% of drug overdoses involved opioids, and 59.8% of these involved synthetic opioids, such as fentanyl (Scholl et al., 2018). This issue has worsened so much that in 2014, the Centers for Disease Control and Prevention added opioid overdose prevention to its list of the top five public health challenges (Kolodny et al., 2015). Development of novel and effective treatments for opioid use disorder will require preclinical research using animal models. Therefore, we developed a mouse model of compulsive-like responding for oral fentanyl that can be used to study the neurobiology of this behavior.
In the present studies, we trained mice to respond for a fentanyl solution that was consumed orally. An important observation in the progression of opioid addiction is that most users begin with prescription opioid pills taken orally. This is exemplified by the fact that four out of five heroin users report that their drug use started with opioid painkillers (Kolodny et al., 2015). Despite this, opioid self-administration studies in animals overwhelmingly use intravenous delivery. However, there may be unique properties of oral administration early in drug use that contribute to the development of dependence and are not captured by intravenous self-administration models. To date, there has been some interest in animal models of oral opioid consumption, which has shown that mice (Enga et al., 2016; Phillips et al., 2019; Wade et al., 2008) and rats (Klein, 2001; Klein et al., 1997; Shaham et al., 1993; Thornton et al., 2000) will reliably respond for oral opioids such as fentanyl and oxycodone in an operant chamber and consume fentanyl in tests of home-cage drinking (Grim et al., 2018, 2017; Shaham et al., 1992; Zanni et al., 2019).
A major goal of the current studies was to determine whether the bitter tastant quinine can be used to model aversion-resistant fentanyl consumption. Animal models of compulsive drug self-administration have used a variety of punishments, including bitter tastants (Wolffgramm, 1991; Darevsky et al., 2019; Halladay et al., 2017; Hopf et al., 2010), foot shock (Chen et al., 2013; Radke et al., 2017), airpuff (Skupio et al., 2017), and intravenous histamine (Holtz et al., 2013; Holtz and Carroll, 2015). Such models have been useful in uncovering the neural mechanisms that underlie compulsive-like reward-seeking and taking (Chen et al., 2013; Halladay et al., 2019; Limpens et al., 2015; Lüscher et al., 2020; Pelloux et al., 2013; Radke et al., 2017, 2015; Seif et al., 2013), though studies investigating punished opioid self-administration are notably lacking. Footshock has been used to punish opioid self-administration in rats (Panlilio et al., 2005, 2003; Peck et al., 2015; Smith and Davis, 1974), but these studies have focused on the use of punishment to study abstinence and relapse. We sought to develop a model of punished opioid self-administration in mice to facilitate future investigations into the neural mechanisms of compulsive-like opioid use.
Quinine has been used by our lab and others to model “aversion-resistant” alcohol drinking in the home cage (Hopf et al., 2010; Radke et al., 2019; Sneddon et al., 2019) and in the operant chamber (Sneddon et al., 2020) and the neural mechanisms underlying alcohol drinking punished with quinine vs. foot shock have been found to be similar (Seif et al., 2013; Halladay et al., 2020; Siciliano et al., 2019). There are a number of advantages to using quinine to punish drug taking. Because it does not induce a conditioned fear response, quinine can be presented repeatedly and responding for a range of concentrations can be assessed. It can also be delivered easily in both the home cage and the operant box. Further, we reasoned that the potent analgesic properties of fentanyl would be less likely to interfere with an animal’s sensitivity to quinine vs. footshock, another common punisher. Thus, we presented C57BL/6J male and female mice with escalating concentrations of quinine in fentanyl during a two-bottle choice, home-cage drinking in the dark task and during a fixed-ratio, operant response task. In both of these paradigms, fentanyl was available orally to model the tendency for human opioid addicts to begin the early stages of their drug use with oral prescribed medications.
Materials and methods
Subjects
C57BL/6J male and female, adult (PND 60+) mice were generated from breeding pairs purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were given standard care and housed in groups of 1–4 per cage (18.4 cm x 29.2 cm x 12.7 cm) in a temperature-controlled room on a 12:12 light:dark cycle. Mice had ad libitum access to food (Rodent Diet 5001 chow; Cincinnati Lab Supply, Cincinnati, OH, USA) and reverse osmosis-filtered (RO) drinking water, unless noted otherwise below. All procedures conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Miami University.
Drugs
Fentanyl citrate (1046538, Henry Schein, Melville, NY, USA) was prepared volume/volume in RO water. Quinine hemisulfate (Sigma, St. Louis, MO, USA) was prepared weight/volume in RO water or in a 10 μg/ml fentanyl solution or a 1% sucrose solution. Fentanyl solutions were prepared weekly. All other solutions were prepared daily before use.
Experiment 1: Fentanyl consumption in a two-bottle choice, drinking in the dark task
We first assessed levels of fentanyl consumption using a drinking in the dark task. Fentanyl was available via two bottles placed on the home cage 3 h after the start of the dark phase of a reverse light:dark cycle (lights on at 7:00 PM/lights off at 7:00 AM). Mice (N = 4 male and 4 female) were transferred to individual cages two weeks prior to the beginning of testing and presented with two drinking bottles of fentanyl citrate (10 μg/ml) on the cage for 11 sessions, for 2 h each session. Body weights were measured daily. Bottles switched sides every session throughout the experiment. To account for spilling, two drinking bottles were placed on empty dummy cages. Consumption for each mouse was measured as the difference in bottle weight before and after the session, minus the average difference in bottle weight for dummy bottles. This was converted to μg/kg of body weight by dividing consumption by the animal’s body weight on that day.
Next, to determine whether fentanyl drinking was resistant to quinine adulteration, quinine was added to the fentanyl bottles. Because some opioids have a bitter taste (though note that the 10 ug/mL concentration of fentanyl has little taste and is not aversive to rats (Carlson, 1989)), we also wanted to determine if mice can detect quinine in the fentanyl solution and discriminate between different concentrations of quinine in fentanyl. Thus, both fentanyl bottles were adulterated with quinine, but at different concentrations. We chose quinine concentrations that are typically used in studies of aversion-resistant alcohol drinking (Lesscher et al., 2010; Sneddon et al., 2019). Quinine (100 μM) was added to one of the bottles and escalating concentrations of quinine were presented in the second bottle (0 μM, 250 μM, and 500 μM). This resulted in three pairings: 1) 0 v. 100 μM quinine in fentanyl, 2) 100 v. 250 μM quinine in fentanyl, and 3) 100 v. 500 μM quinine in fentanyl (Figure 1A). Each pair of quinine concentrations was presented for 3 sessions.
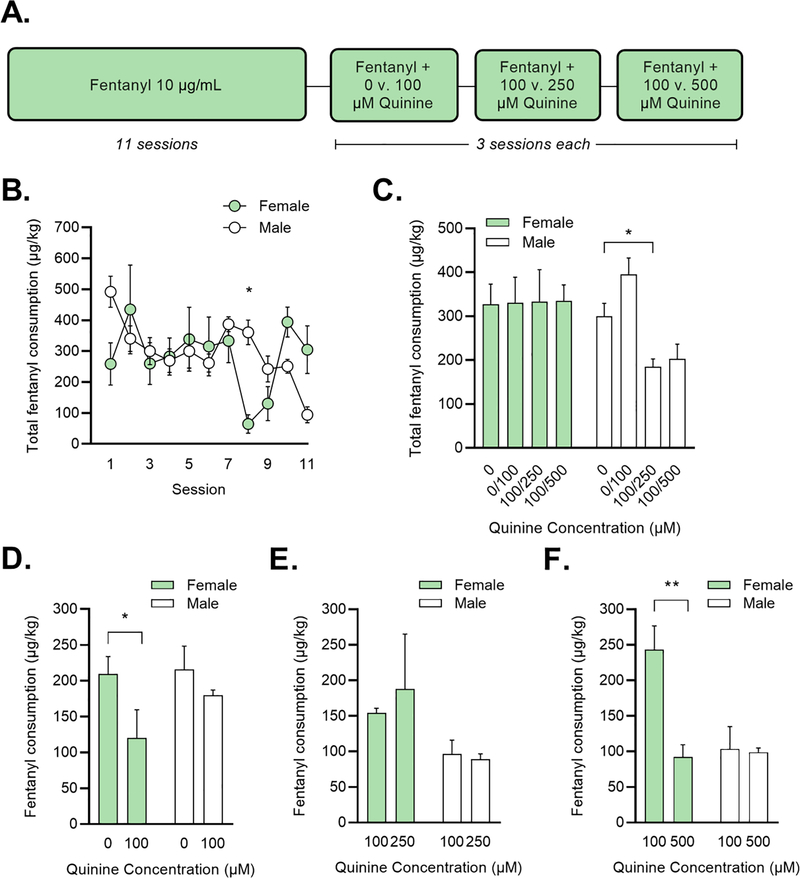
Figure 1: Fentanyl self-administration in a home-cage drinking in the dark task.
(A) Mice were given two bottles of fentanyl (10 μg/mL) for 2 h/day. After 11 sessions of drinking, quinine was added to the bottles. (B) Total fentanyl consumption in female (=green) and male (=white) mice over the first 11 sessions. *p < 0.05 vs. females on that session (Holm-Sidak test). (C) Total fentanyl consumption in female and male mice during quinine sessions. *p < 0.05 vs. 0-μM sessions (Dunnett’s test). (D-F) Fentanyl consumption from each bottle during quinine sessions. *p < 0.05, **p < 0.01 vs. 0-μM sessions (Dunnett’s test).
Experiment 2: Responding for oral fentanyl in the operant chamber
Operant response training and testing
To study aversion-resistant responding for oral fentanyl in the operant box, mice were trained to nose poke for a fentanyl solution. We based our methods off those described by Wade et al., 2008. Training and testing were conducted in a chamber measuring 15.24 × 13.34 × 12.7 cm (ENV-307A, Med Associates, Fairfax, VT, USA) housed in a sound- and light-attenuating box (ENV-022V). On one end of the chamber was a receptacle and cup for reward delivery (ENV303RMA-3; ENV-303RL), a house light (ENV-315M), and 2 nose-poke holes (ENV-313M) (left = active, right = inactive throughout all sessions). The floor was made of 19 metal rods with 0.79 cm of space between each rod (ENV-307A-GFW). Boxes were connected to a computer using Med-PC® V Software Suite (SOF-736) for data collection.
For response training, mice began on session 1 with one food pellet (F05684, Bio-Serv, Flemington, NJ, USA) waiting in the reward receptacle. The nose-poke holes and reward receptacle were illuminated and the house light was off. Fentanyl citrate (10 μg/ml) was available on a fixed-ratio 1 (FR1) schedule for 2 h. Each response on the active nose-poke hole resulted in delivery of 50 μL of fentanyl solution and sounding of a 2-s, 65-dB tone. Responses at the inactive nose-poke hole had no consequence. Fentanyl solution was dispensed into the cup from a single-speed syringe pump (PHM-100, Med Associates) via a 20-mL syringe connected to polyethylene tubing. A 5-s timeout, during which the nose-poke holes were not illuminated and no reward could be earned, followed reward delivery. These sessions were repeated once per day, 5 days/week. Sessions were run during the light phase of the light:dark cycle (lights off at 7:00 PM), between 12:00 and 5:00 PM. Food restriction (to 85% of free-feeding body weight) was used to motivate responding. Mice were fed once per day immediately after the self-administration session.
Mice (N = 12 male and 11 female) self-administered fentanyl for a total of 15 sessions (Figure 2A). After session 15, a series of escalating concentrations of quinine was added to the fentanyl solution. Each concentration of quinine (0 μM, 100 μM, 250 μM, 500 μM) was presented for three days.
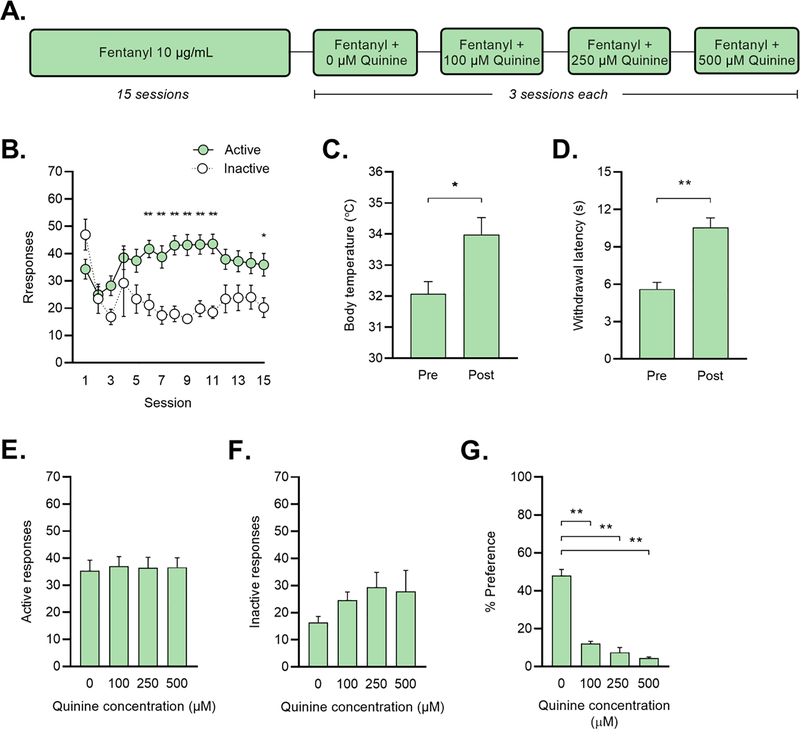
Figure 2: Fentanyl self-administration in an operant-response task.
(A) Mice responded for fentanyl (10 μg/mL) for 2 h/day on an FR1 schedule of reinforcement. After 15 sessions, escalating concentrations of quinine were added to the fentanyl solution. (B) Responses for fentanyl at the active (=green) and inactive (=white) nose-poke holes. *p < 0.05, **p < 0.01 vs. inactive responses on that session (Holm-Sidak test). (C) Body temperature and (D) paw withdrawal-latency in the hot plate test before (=Pre) and immediately after (=Post) the self-administration session *p < 0.05, **p < 0.01 vs. Pre (paired t-test). (E) Active responses and (F) inactive responses during quinine sessions. (G) Consumption of quinine in water in a 48-h two-bottle preference test. **p < 0.01 vs. 0-μM (Dunnett’s test).
Body temperature
Mu opioid receptor activation has been shown to induce hyperthermia (Rawls et al., 2003; Rawls and Benamar, 2011). Thus, to verify consumption of fentanyl, a subset of mice (N = 4 male, 4 female) were examined for changes in body temperature. This was done immediately before and after self-administration session 14 using a rectal thermometer (34–1401, Harvard Apparatus, Holliston, MA, USA).
Hot plate test for nociception
Given fentanyl’s analgesic effects, fentanyl consumption was also verified in the same subset of mice (N = 4 male, 4 female) by changes in paw withdrawal latency using a hot plate test. The test was conducted on session 16 at least one hour before the start of the self-administration sessions, to avoid the possibility of pain-induced escalation of intake. Mice were placed on a custom-built 55°C plate and latency in seconds for the mouse to retract its feet from the plate was recorded. A second test was conducted immediately after the self-administration session on the same day.
Two-bottle choice test of quinine sensitivity
Two weeks after cessation of fentanyl self-administration, all mice (N = 23) went through a two-bottle choice task in the home cage as a measure of quinine sensitivity. Two water bottles were placed on each animal’s home cage for a period of 48 h, one with unaltered RO drinking water and the other with escalating concentrations of quinine (0 μM, 100 μM, 250 μM, 500 μM) in drinking water. Every 24 h, bottle positions were switched to limit side bias. Two dummy cages were equipped with bottles to account for spillage and evaporation. Quinine preference in the two-bottle home cage drinking test was calculated as percent preference [= (quinine consumption/total consumption)* 100].
Experiment 3: Effects of quinine on fentanyl-responding early in training
To assess whether quinine could reduce responding for fentanyl when presented early in training, we trained a second group of mice to self-administer fentanyl in the operant chamber, using procedures identical to those described above. Mice self-administered fentanyl citrate (10 μg/ml) for a total of 15 sessions. One group of mice (N = 4 male and 4 female) responded for fentanyl alone (=fentanyl). A second group of mice (N=9 male and 7 female) self-administered fentanyl (10 μg/ml) with the bitter tastant quinine (500 μM) added to the solution (=fentanyl+quinine) (Figure 3A). For the fentanyl+quinine group, quinine was mixed with fentanyl on all 15 self-administration sessions.
Figure 3: Fentanyl + quinine self-administration in an operant-response task.
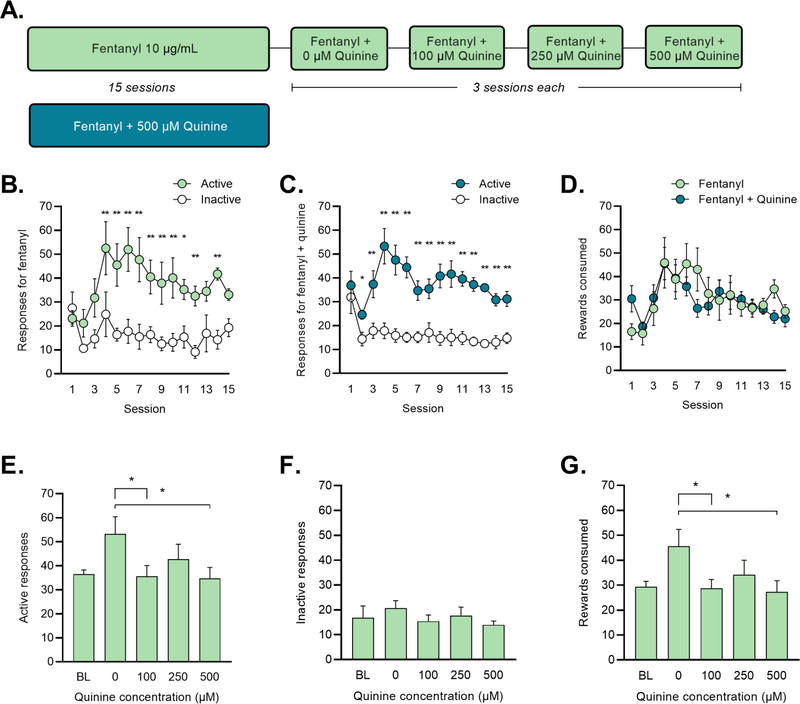
(A) Mice responded for fentanyl (=green) or fentanyl + 500-μM quinine (=blue) for 2 h/day on an FR1 schedule of reinforcement. For the fentanyl-group, escalating concentrations of quinine were added to the fentanyl solution after 15 sessions. (B-C) Active (=green) and inactive (=white) responses for (B) fentanyl and (C) fentanyl + quinine. *p < 0.05, **p < 0.01 vs. inactive responses on that session (Holm-Sidak test). (D) Rewards consumed in mice responding for fentanyl (=green) and fentanyl + quinine (=blue). (E) Active responses, (F) inactive responses, and (G) rewards consumed during quinine sessions. *p < 0.05 vs. 0-μM (Dunnett’s test).
For this experiment, we also recorded any solution remaining in the drinking cup at the end of the session and verified that the reward solution does not leak or evaporate from the cup during the course of an operant session. Although this measure should not be interpreted as a strict measure of consumption, estimating the rewards consumed in the operant response task allowed us verify that mice were not responding for quinine-adulterated fentanyl without consuming it.
Experiment 4: Effects of quinine on sucrose responding in the operant chamber
Finally, we tested whether the selected concentrations of quinine reduce operant responding for a non-drug reward (1% sucrose solution). Mice (N = 8 male and 8 female) self-administered sucrose (1%) in the operant chamber for 15 sessions, exactly as described above for fentanyl self-administration. Next, a series of escalating concentrations of quinine was added to the fentanyl solution (0 μM, 100 μM, 250 μM, 500 μM) with each concentration presented for 3 days.
Statistical analysis
For all experiments, data were first analyzed with sex as a factor. In Experiments 2 and 3, no significant effects of sex were found so data were collapsed across this factor for subsequent analysis and presentation. For quinine sessions, data were averaged over the three days of presentation for each concentration.
Data from two-bottle choice experiments are presented as consumption (=μg of fentanyl consumed per kg of bodyweight) or % preference. These data were analyzed using two-factor mixed-design ANOVA with concentration or session as the within-subjects factor and sex as the between-subjects factor. Post-hoc Holm-Sidak or Dunnett’s multiple comparisons tests were used to assess differences between sexes or quinine concentrations, as appropriate.
For operant response experiments, responses (active and inactive) were summed across the entire session. Active responses excluded any responses made during the time out following reward delivery. Two-way repeated measures (RM) ANOVA with session and response as within-subjects factors was used to analyze behavior on sessions 1–15. Responses on quinine sessions were analyzed using one-way RM ANOVA. For Experiment 3, the number of rewards consumed was calculated (= (rewards earned) - (number of 50-μL rewards left in the cup at the end of the session) and compared between mice responding for fentanyl vs. fentanyl+quinine using two-factor mixed-design ANOVA with session as the within-subject factor and treatment as the between-subjects factor. Consumption as a factor of body weight was also calculated and analyzed, but as the results were unaltered these data are not shown. Post-hoc Holm-Sidak or Dunnett’s multiple comparisons tests were used to assess differences between responses or quinine concentrations.
All data are expressed as mean ± standard error of the mean. The alpha level was set at p < 0.05 and all statistical results meeting this threshold are reported in the text and on the figures. Statistical analyses were performed with GraphPad Prism, v8.4.
Results
Experiment 1: Sex differences in quinine effects on fentanyl drinking in a two-bottle choice task
We began by conducting a two-bottle choice task to determine whether mice could detect quinine in fentanyl. For 11 sessions, mice were presented with unaltered fentanyl (10 μg/ml) in drinking water from two bottles on the home cage (Figure 1A). The average consumption per 2-h sessions was 313.7 μg/kg. Two-factor mixed-design ANOVA revealed a significant effect of session (F (10, 60) = 3.448, p = 0.001) and a significant sex x session interaction (F (10, 60) = 3.448, p < 0.001). On session 8, females consumed significantly less than males (Holm-Sidak, p = 0.016) (Figure 1B) but consumption did not differ between the sexes on any other session. Total intake summed across all 11 session was also not different between the sexes (male = 3299.08 ± 324.30 μg/kg; female = 3601.81 ± 503.94 μg/kg).
Next, we introduced escalating concentrations of quinine (0 μM - 500 μM) to the bottles. We were interested in whether males and females would differ in their total consumption from both bottles after quinine was added. The experimental design allowed us to compare consumption from the two bottles in the following manner: 0 μM vs. 100 μM, 100 μM vs. 250 μM, and 100 μM vs. 500 μM. Two-factor mixed-design ANOVA revealed a significant effect of quinine concentration (F (3, 18) = 4.617, p = 0.015) and a significant sex x concentration interaction (F (3, 18) = 5.045, p = 0.010) (Figure 1C). Females did not decrease their total fentanyl consumption at any level of quinine; On the contrary, compared to consumption of fentanyl with no quinine (0 μM), males consumed significantly less fentanyl when one bottle had 100 μM quinine and the other had 250 μM (Dunnett’s test, p = 0.048). In other words, females continue to consume fentanyl in the presence of the selected concentrations of quinine, while males suppress their consumption at higher quinine levels.
We also analyzed differences in consumption between the two fentanyl bottles. With the 0 μM vs. 100 μM comparison, a two-factor mixed-design ANOVA revealed a significant main effect of concentration (F (1, 6) = 9.590, p = 0.021). Females drank more from the 0 μM bottle v. the bottle with 100 μM quinine (Holm-Sidak, p = 0.041) while males did not prefer either bottle (Figure 1D). For 100 μM vs. 250 μM, consumption was similar between bottles for both males and females (Figure 1E). At the 100 μM vs. 500 μM comparison, there were significant main effects of sex (F (1, 6) = 7.195, p = 0.036) and quinine concentration (F (1, 6) = 10.130, p = 0.019) and a significant sex x concentration interaction (F (1, 6) = 8.906, p = 0.024). Post-hoc Holm-Sidak comparisons revealed that females preferred the bottle with 100 μM quinine and suppressed their consumption from the bottle with 500 μM (p = 0.010) (Figure 1F).
Finally, because differences in fentanyl consumption at baseline could influence the development of quinine-resistant drinking, we correlated consumption of quinine-free fentanyl with intake at each of the three pairs of quinine concentrations. Neither total intake across sessions 1–11 nor intake during the final three quinine-free sessions correlated with consumption at any quinine concentration.
Experiment 2: Quinine does not reduce responding for fentanyl in an operant response task
In a naive cohort of mice, we also examined fentanyl responding in an operant paradigm. Mice administered fentanyl for 15 days, after which a series of escalating concentrations of quinine (0 μM-500 μM) was added for three days each (Figure 2A). In this task, active responses were classified as responses on the side of the chamber that led to fentanyl delivery while inactive responses resulted in nothing. During the initial 15 sessions, we observed a significant main effect of session (F (14, 308) = 1.994, p = 0.018), response (F (1,22) = 22.820, p < 0.001), and a significant session x response interaction (F (14, 308) = 3.819), p < 0.001) (Figure 2B). Post-hoc Holm-Sidak comparisons revealed a significant difference in active v. inactive responses on sessions 6–11 (p < 0.01) and on session 15 (p < 0.05) (Figure 2B).
In a subset of mice (n = 8), we also measured body temperature and pain sensitivity as confirmatory measures of fentanyl consumption. A paired t-test revealed a significant increase in body temperature after session 14 (p = 0.037) (Figure 2C). A paired t-test also revealed a significant increase in paw withdrawal latency after session 16 (p = 0.001) (Figure 2D). Next, we measured active and inactive responses over quinine sessions (0 μM sessions 16–18, 100 μM sessions 19–21, etc.). One-way ANOVA revealed no significant effects of concentration on these measures (Figures 2E–F).
Two weeks after completion of the operant experiment, we confirmed that mice trained to self-administer fentanyl in the operant box find the selected concentrations of quinine aversive. Using a 48-h two-bottle choice task in the home cage, quinine concentrations (0–500 μM) were presented in an escalating manner. Mice dramatically reduced their total water consumption from the bottle with quinine. A one-way ANOVA exhibited a significant effect of quinine concentration (F (3, 66) = 112.3, p < 0.001) (Figure 2G). Post-hoc Dunnett’s multiple comparisons found a significant difference in percent preference when comparing 0 μM to all other quinine concentrations (p < 0.001 for all, Figure 2G).
Experiment 3: Quinine early in training does not affect fentanyl responding
Quinine-resistant fentanyl self-administration was observed in both the drinking in the dark task and the operant response task. To determine whether aversion-resistant behavior was dependent on fentanyl exposure history, we trained one group of mice to respond for fentanyl and another to respond for fentanyl mixed with 500 μM quinine for 15 sessions (Figure 3A). Mice in the fentanyl group earned an average of 916.2 μg/kg in the 2-h session. The pattern of active and inactive responses was similar in both groups (Figure 3B–C). In the fentanyl group, a two-way RM ANOVA revealed significant main effects of session (F (14, 98) = 2.001, p = 0.025) and response (F (1, 7) = 20.530, p = 0.003) and a session x response interaction (F (14, 98) = 1.921, p = 0.033) (Figure 3B). Post-hoc Holm-Sidak multiple comparisons found that mice responding for fentanyl made significantly more active vs. inactive responses on sessions 4–12 and 14 (p < 0.01 for all). A two-way RM ANOVA examining responses in the fentanyl+quinine group revealed significant main effects of session (F (14, 210) = 2.484, p = 0.003) and response (F (1, 15) = 110.800, p < 0.001) and a session x response interaction (F (14, 210) = 3.766, p < 0.001). Post-hoc Holm-Sidak multiple comparisons found that mice responding for fentanyl+quinine made significantly more active vs. inactive responses on all sessions except session 1 (p < 0.05 for session 2, p < 0.01 for all other sessions) (Figure 3C). When responding during the 2-h session was analyzed as the number of active responses made in 2-min intervals, a steady pattern of responding throughout the sessions was evident. On average, responses were made in about half of the 60 2-min intervals, as evidenced by the number of 2-min intervals with no responses recorded (baseline sessions: 30.08 ± 3.19, 0-μM sessions: 26.13 ± 3.39, 100-μM sessions: 32.08 ± 3.76, 250-μM sessions: 32.42 ± 3.47, 500-μM sessions: 36.04 ± 3.77). Intervals with responses were distributed throughout the 2-h session. These data suggest that mice consistently responded for fentanyl reinforcement throughout the 2-h sessions.
Another goal of this experiment was to confirm that mice were consuming quinine-adulterated fentanyl delivered to the operant box. For both fentanyl and fentanyl+quinine groups, we quantified the number of rewards consumed, as described in the Methods. A two-way RM ANOVA examining rewards consumed in both groups found only a significant main effect of session (F (14, 308) = 4.408, p < 0.001). Thus, there were no differences between groups in the number of rewards consumed throughout the 15 sessions of training (Figure 3D).
Mice administering fentanyl only were next tested for aversion resistance by adding escalating concentrations of quinine to the solution for three days each (exactly as in Experiment 2) (Figure 3A). When we investigated active responses at each quinine concentration (0, 100, 250, and 500 uM), a one-way ANOVA exhibited a significant effect of concentration (F (3, 21) = 4.563, p = 0.013) (Figure 3E). A post-hoc Dunnett’s multiple comparisons test comparing all concentrations to 0 μM found a significant difference between active responses at 100 μM (p = 0.014) and 500 μM (p = 0.010). A one-way ANOVA examining inactive responses was not significant (Figure 3F). A one-way ANOVA examining rewards consumed found a significant effect of concentration (F (3, 21) = 4.876, p = 0.010) (Figure 3G). A post-hoc Dunnett’s multiple comparison test comparing all concentrations to 0 μM found a significant difference between rewards consumed at 100 μM (p = 0.013) and 500 μM (p = 0.007). After observing that responding on the 0 μM quinine sessions was higher than the preceding sessions, we also compared behavior on the quinine sessions (100, 250, and 500 μM) to the average of sessions 13–15 (=baseline). One-way ANOVAs examining active responses, inactive responses, and rewards consumed on these sessions were not significant.
Experiment 4: Quinine suppresses sucrose responding in the operant-response task
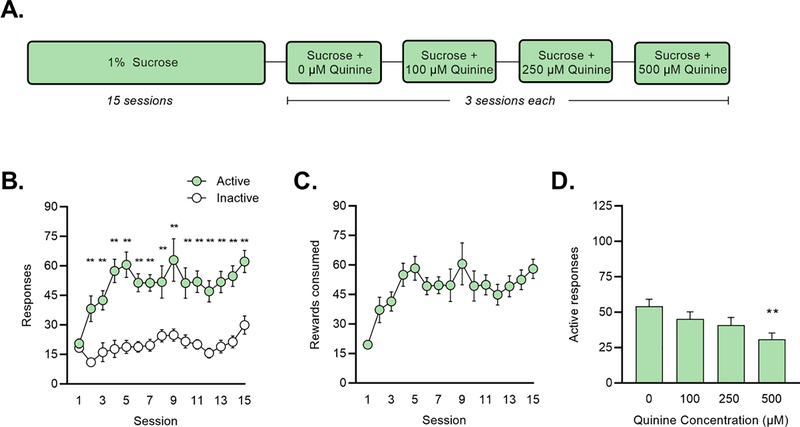
Over the first 15 sessions, mice responded for and consumed the 1% sucrose solution at rates similar to, though slightly higher than, those observed for fentanyl (Figure 4A–B). For responses, a two-way RM ANOVA revealed significant main effects of session (F (14,210) = 4.765, p < 0.001) and response (F (1,15) = 65.090, p < 0.001). Post-hoc Holm-Sidak comparisons revealed a significant difference in active v. inactive responses on sessions 2–15 (p < 0.01 for all sessions). During quinine sessions, a one-way ANOVA examining responses found a significant effect of quinine concentration (F (4,60) = 7.991, p < 0.001). Post hoc Dunnett’s tests comparing responses at each concentration to baseline (average of sessions 13–15) found that responding was reduced at 250 μM (p = 0.022) and 500 μM (p < 0.001) (Figure 4C).
Figure 4: Sucrose + quinine self-administration in an operant-response task.
(A) Mice responded for 1% sucrose for 2 h/day on an FR1 schedule of reinforcement. Escalating concentrations of quinine were added to the sucrose solution after 15 sessions. (B) Active (=green) and inactive (=white) responses for sucrose. *p < 0.05, **p < 0.01 vs. inactive responses on that session (Holm-Sidak test). (C) Sucrose rewards consumed. (D) Active responses during quinine sessions.**p < 0.01 vs. 0-μM (Dunnett’s test).
Discussion
In both the home-cage two-bottle choice task and the operant response task, mice consumed fentanyl solution and demonstrated aversion-resistant behavior when quinine was added to the solution. Mice were able to detect quinine in fentanyl, as demonstrated by a reduction in total fentanyl consumption in males when 250-μM quinine was introduced in the two-bottle choice task and preference for the bottle with the lower quinine concentration in females. Both males and females tolerated 100-μM quinine in fentanyl in the two-bottle choice task, although females only did so when a quinine-free alternative was not available. In the operant task, mice robustly responded for and consumed fentanyl and demonstrated quinine-resistance at all concentrations tested. This was true whether quinine was added on the first session of self-administration or after 2.5 weeks of training with fentanyl alone. Measurements of body temperature and paw withdrawal latency, a measure of pain sensitivity, confirmed consumption of fentanyl in the operant task. Quinine-suppression of sucrose responding demonstrates that quinine is an effective punisher under the training parameters used in this study and that aversion-resistance was specific to fentanyl. Finally, we demonstrated that mice trained to respond for fentanyl in the operant box will avoid the selected concentrations of quinine in water.
Sex-dependent differences in responding for quinine-adulterated fentanyl were observed in the two-bottle drinking task. Females preferred to consume fentanyl solutions that contained lower levels of quinine. Specifically, female mice preferred quinine-free fentanyl vs. fentanyl adulterated with 100-μM quinine and preferred fentanyl with 100-μM quinine to a solution containing 500 μM. In contrast, males did not exhibit preferences for different concentrations of quinine in fentanyl and instead reduced consumption from both bottles equally when the 250-μM concentration was introduced. Further, while total consumption of fentanyl for females did not change at any point throughout the experiment, males consumed less fentanyl once both bottles contained quinine. These drinking patterns suggest a sex-dependent manner of consumption in which females will seek out the least aversive source of fentanyl in order to keep consumption levels constant, while males do not seem to use this strategy. Studies of quinine-resistant alcohol drinking suggest that males can discriminate between the concentrations used in this study (e.g., male mice reduce their alcohol consumption when presented with quinine concentrations greater than 250 μM but not 100 μM; Sneddon et al. 2019). It is possible that males generalize more between the two bottles or are less likely to continuing seeking drug after encountering the more aversive quinine concentration. Overall, these results suggest that both males and females will tolerate 100-μM quinine in fentanyl and that females may be more resistant to quinine aversion than males. Greater vulnerability in female mice would concur with recent examinations of sex differences in aversion-resistant alcohol drinking (Fulenwider et al., 2019; Radke et al., 2019; Xie et al., 2019; Sneddon et al. 2020). Such behavioral differences are unlikely to be due to sex differences in taste sensitivity, as we have shown here in Experiments 2 and 4 and in prior studies (Radke et al., 2019; Sneddon et al., 2019; Sneddon et al., 2020) that male and female rodents are equally sensitive to quinine in water and sucrose.
The total amount of fentanyl consumed did not differ by sex in either task. In the operant response experiments, this was true whether or not the total fentanyl consumed was corrected for body weight. In contrast, higher levels of self-administration of oral oxycodone (Phillips et al., 2019; Zanni et al., 2019) and intravenous heroin and morphine (Cicero et al., 2003) has been observed in female rodents, though at least one study found similar levels of oral morphine ingestion in C57BL/6J male and female mice (Forgie et al., 1988). Based on our results, it appears that oral fentanyl does not engender a sex difference in consumption at the 10 μg/mL concentration used. We also did not observe a sex difference in quinine-resistant behavior in our operant task. Both males and females continued to respond for and consume fentanyl at the highest concentration of quinine tested. We did not test the 250 and 500 μM concentrations of quinine alone in the two-bottle task, but decreased consumption in males when those concentrations were added to one of the bottles suggests that the operant task may induce stronger resistance to quinine aversion, and highlights the importance that the behavioral paradigm plays in measures of aversion resistance.
One striking difference between the two paradigms described here is the difference in the overall level of fentanyl exposure mice received. Mice responding for fentanyl in the operant box earned almost three times as much drug as was consumed in the two-bottle fentanyl drinking task. One reason for the differences in the total amount of fentanyl consumed in the operant-response vs. the two-bottle task may be the rate of drug consumption. The two-bottle drinking in the dark task is a model of binge drinking (Thiele et al., 2014), and rapid consumption may promote intoxication and constrain the total amount of drug mice can consume in the 2-h session. Investigators wishing to use home cage drinking procedures for opioid self-administration may find that limiting access to the drinking bottle or providing access during a different time point in the light-dark cycle is necessary to maximize intake. Lowering the concentration of fentanyl may also be an effective strategy. The concentration used here (10 μg/mL) was selected to facilitate comparison between the two-bottle drinking and operant response tasks. Further work will be necessary to characterize intake patterns across a range of oral fentanyl concentrations.
The experimental design used for the home cage drinking studies (Experiment 1) does differ from most studies of quinine-resistant alcohol drinking in that mice did not have a non-drug alternative (e.g., water) available during the drinking session. The current data, along with results of studies examining aversion-resistance using operant response tasks (Radke et al., 2017; Halladay et al., 2017; Sneddon et al., 2020), suggest that the choice of water is not necessary for compulsive-like responding to develop so long as other important factors such as intermittent and voluntary access are preserved (Wolffgramm, 1991).
In Experiment 2, quinine did not reduce fentanyl responding at any concentration in the operant box. This result was also observed in Experiment 3 when sessions 13–15 were used as a baseline. It is important to note that responding for the 100- and 500-μM quinine concentrations was reduced compared to sessions 16–18, which were originally intended to serve as 0-μM comparison sessions. The higher level of responding observed on sessions 16–18 (v. preceding sessions) unfortunately makes the results of this experiment somewhat inconclusive. However, when considered alongside the results of Experiment 2 (in which more animals were tested) and the lack of an effect of quinine in the fentanyl+quinine group in Experiment 3, it is reasonable to conclude that mice demonstrate aversion-resistant responding for fentanyl at all quinine concentrations. Importantly, mice maintain their sensitivity to quinine after fentanyl self-administration, as evidenced by reduced consumption of quinine in water in the 48-h two-bottle task in Experiment 2. The results of Experiment 4 demonstrate that quinine reduces operant responding for a 1% sucrose solution, a result that concurs with our previous demonstration that quinine suppresses both ethanol and sucrose responding during a 30-min, FR3 session (Sneddon et al., 2020). These results demonstrate that quinine is an effective punisher in this operant task and suggest there is something unique about fentanyl (vs. ethanol or sucrose) reinforcement or intoxication that induces greater resistance to aversion.
Although exposure levels have been shown to affect the development of aversion-resistant alcohol drinking (Hopf et al., 2010; Radke et al., 2019, 2017; Vendruscolo et al., 2012) and addiction-like cocaine self-administration behaviors (Deroche-Gamonet et al., 2004), exposure history does not seem to have influenced the development of aversion resistance in the current study. In Experiment 3, the behavior of mice responding for fentanyl+quinine on sessions 1–15 was not different from those self-administering fentanyl alone; both the number of responses and rewards consumed were almost identical in the two groups. This result suggests that naive mice are as resistant to quinine aversion in the operant box as mice with a history of fentanyl exposure. Such a rapid induction of aversion-resistance may be unique to the C57BL/6 strain of mice, which are reward-preferring (Belknap et al., 1993; Lewis et al., 2005; Pelz et al., 1973) and exhibit quinine-resistant alcohol drinking after only one exposure to alcohol (Lei et al., 2016).
Fentanyl was delivered to the operant box via a syringe connected to a drinking cup in the chamber and we observed that mice did not always consume the entire amount of fentanyl earned via their responses at the nose-poke hole. To assess whether this influenced our results, we estimated the number of rewards consumed by collecting the solution left in the drinking cup at the end of the session in Experiment 3. On average, we found that approximately seven 50-μL rewards remained at the end of the session and this number did not change with the addition of quinine to the solution. It is not clear whether fentanyl accumulated in the cup over the session (i.e., mice did not drink the entire 50-μL when each reward was delivered) or if mice responded for rewards that they did not consume at all. In either case, the results of this analysis along with the physiological measures used to verify consumption in Experiment 2, suggest that mice do consume fentanyl earned in the operant box and that quinine reduces neither responding nor consumption. Further, while the physiological measures do not provide a precise measurement of intoxication, they do suggest that mice were exposed to meaningful levels of fentanyl. For example, one recent study of oral oxycodone drinking in rats demonstrated escalated intake, signs of dependence, and measureable serum levels of oxycodone at consumption levels that produced no change in paw withdrawal latency in the hot plate test (Zanni et al. 2019). Further, studies in rodents have found changes in nociceptive thresholds (Minami et al. 2009; Waxman et al. 2009; Eastwood and Phillips 2014; Fujii et al. 2018; Varshneya et al. 2019) and body temperature (Vujovic et al. 2013) following injection of fentanyl at doses that also produce locomotor activation (Eastwood and Phillips 2014; Varshneya et al. 2019) and conditioned place preference (Mucha and Herz 1985; Finlay et al. 1988; Miller and Nation 1997; Sustkova-Fiserova et al. 2019) (doses ranging from 0.025 to 0.4 mg/kg).
The inability to precisely measure consumption in the operant box is a limitation of these studies. Indeed, one potential explanation for the disparate results in the two-bottle and operant response tasks is that quinine produces relatively small decreases in fentanyl consumption that cannot be captured in the operant task. The measure of rewards consumed was included to verify that mice were not persisting in responding without actually consuming the fentanyl solution following quinine adulteration, but this metric is not a sensitive measure of consumption. Considering evidence that consumption of alcohol better predicts blood ethanol levels vs. operant responses, increasing the precision of the consumption measurement in our task is critical for future studies (Blegen et al. 2018). Combining precise measures of lick rate and consumption (e.g., with lickometers in the operant chamber) with measures of responding would improve the utility of this paradigm.
Using oral fentanyl to study opioid addiction has some benefits over intravenous self-administration. Oral self-administration is less technically challenging, particularly in mice, and models the predominant mode of exposure responsible for the initiation of human opioid use disorder. Use of high potency opioids like fentanyl is advantageous as they can be delivered orally without the bitter taste associated with other opiate drugs (e.g., morphine). The results of this study demonstrate that C57Bl/6J mice will self-administer higher levels of fentanyl in an operant chamber vs. in a home cage drinking task but demonstrate aversion-resistant drinking in both paradigms. Further, sex differences emerged when mice drank fentanyl in the home cage but not in the operant chamber. Thus, both models have benefits that may suit a variety of experimental questions. These models will be useful in evaluating the neural mechanisms that contribute to the development of opioid use disorder and compulsive-like opioid taking.
Acknowledgements
We are grateful to Marissa Muench and Kristen Schuh for technical assistance. Research supported by the College of Arts and Sciences, Department of Psychology, Committee on Faculty Research, and the Office of Research and Innovation at Miami University. The authors declare no competing interests in this work.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Belknap JK, Crabbe JC, Riggan J, O’Toole LA, 1993. Voluntary consumption of morphine in 15 inbred mouse strains. Psychopharmacology 112, 352–358. [DOI] [PubMed] [Google Scholar]
- Blegen MB, Daniel da Silva ES, Bock R, Morisot N, Ron D, Alvarez VA, 2017. Alcohol operant self-administration: Investigating how alcohol seeking behaviors predicts drinking in mice using two operant approaches. Alcohol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson KR, 1989. Taste vs. CNS effects in voluntary oral opiate intake: studies with a novel device and technique. Pharmacol. Biochem. Behav 34, 419–423. [DOI] [PubMed] [Google Scholar]
- Chen BT, Yau H-J, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A, 2013. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496, 359–362. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER, 2003. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol. Biochem. Behav 74, 541–549. [DOI] [PubMed] [Google Scholar]
- Darevsky D, Gill TM, Vitale KR, Hu B, Wegner SA, Hopf FW, 2019. Drinking despite adversity: behavioral evidence for a head down and push strategy of conflict-resistant alcohol drinking in rats. Addict. Biol 24, 426–437. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV, 2004. Evidence for addiction-like behavior in the rat. Science 305, 1014–1017. [DOI] [PubMed] [Google Scholar]
- Eastwood EC, Phillips TJ, 2014. Opioid sensitivity in mice selectively bred to consume or not consume methamphetamine. Addict. Biol 19, 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enga RM, Jackson A, Damaj MI, Beardsley PM, 2016. Oxycodone physical dependence and its oral self-administration in C57BL/6J mice. Eur. J. Pharmacol 789, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW, 2005. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci 8, 1481–1489. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Jakubovic A, Phillips AG, Fibiger HC, 1988. Fentanyl-induced conditional place preference: lack of associated conditional neurochemical events. Psychopharmacology 96, 534–540. [DOI] [PubMed] [Google Scholar]
- Forgie ML, Beyerstein BL, Alexander BK, 1988. Contributions of taste factors and gender to opioid preference in C57BL and DBA mice. Psychopharmacology 95, 237–244. [DOI] [PubMed] [Google Scholar]
- Fujii K, Koshidaka Y, Adachi M, Takao K, 2019. Effects of chronic fentanyl administration on behavioral characteristics of mice. Neuropsychopharmacol Rep 39, 17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulenwider HD, Nennig SE, Price ME, Hafeez H, Schank JR, 2019. Sex differences in aversion-resistant ethanol intake in mice. Alcohol Alcohol 54, 345–352. [DOI] [PubMed] [Google Scholar]
- Grim TW, Park SJ, Schmid CL, Laprairie RB, Cameron M, Bohn LM, 2018. The effect of quinine in two bottle choice procedures in C57BL6 mice: Opioid preference, somatic withdrawal, and pharmacokinetic outcomes. Drug Alcohol Depend. 191, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim TW, Schmid CL, LaPrairie RB, Park J-H, Lin L, Cameron MD, Bohn LM, 2017. Preference, dependence, and pharmacokinetic outcomes in an opioid two bottle choice paradigm. The FASEB Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay LR, Kocharian A, Holmes A, 2017. Mouse strain differences in punished ethanol self-administration. Alcohol 58, 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay LR, Kocharian A, Piantadosi PT, Authement ME, Lieberman AG, Spitz NA, Coden K, Glover LR, Costa VD, Alvarez VA, Holmes A, 2020. Prefrontal regulation of punished ethanol self-administration. Biol. Psychiatry 87, 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Anker JJ, Regier PS, Claxton A, Carroll ME, 2013. Cocaine self-administration punished by i.v. histamine in rat models of high and low drug abuse vulnerability: effects of saccharin preference, impulsivity, and sex. Physiol. Behav 122, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Carroll ME, 2015. Cocaine self-administration punished by intravenous histamine in adolescent and adult rats. Behav. Pharmacol 26, 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Chang S-J, Sparta DR, Bowers MS, Bonci A, 2010. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol. Clin. Exp. Res 34, 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, 2007. Brain reward systems and compulsive drug use. Trends Pharmacol. Sci 28, 135–141. [DOI] [PubMed] [Google Scholar]
- Klein LC, 2001. Effects of adolescent nicotine exposure on opioid consumption and neuroendocrine responses in adult male and female rats. Exp. Clin. Psychopharmacol 9, 251–261. [DOI] [PubMed] [Google Scholar]
- Klein LC, Popke EJ, Grunberg NE, 1997. Sex differences in effects of predictable and unpredictable footshock on fentanyl self-administration in rats. Exp. Clin. Psychopharmacol 5, 99–106. [DOI] [PubMed] [Google Scholar]
- Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, Alexander GC, 2015. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu. Rev. Public Health 36, 559–574. [DOI] [PubMed] [Google Scholar]
- Lei K, Wegner SA, Yu J-H, Simms JA, Hopf FW, 2016. A single alcohol drinking session is sufficient to enable subsequent aversion-resistant consumption in mice. Alcohol 55, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HMB, van Kerkhof LWM, Vanderschuren LJMJ, 2010. Inflexible and indifferent alcohol drinking in male mice. Alcohol. Clin. Exp. Res 34, 1219–1225. [DOI] [PubMed] [Google Scholar]
- Lewis SR, Ahmed S, Dym C, Khaimova E, Kest B, Bodnar RJ, 2005. Inbred mouse strain survey of sucrose intake. Physiol. Behav 85, 546–556. [DOI] [PubMed] [Google Scholar]
- Limpens JHW, Damsteegt R, Broekhoven MH, Voorn P, Vanderschuren LJMJ, 2015. Pharmacological inactivation of the prelimbic cortex emulates compulsive reward seeking in rats. Brain Res 1628, 210–218. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Robbins TW, Everitt BJ, 2020. The transition to compulsion in addiction. Nat. Rev. Neurosci 21, 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DK, Nation JR, 1997. Chronic cadmium exposure attenuates the conditioned reinforcing properties of morphine and fentanyl. Brain Res 776, 162–169. [DOI] [PubMed] [Google Scholar]
- Minami K, Hasegawa M, Ito H, Nakamura A, Tomii T, Matsumoto M, Orita S, Matsushima S, Miyoshi T, Masuno K, Torii M, Koike K, Shimada S, Kanemasa T, Kihara T, Narita M, Suzuki T, Kato A, 2009. Morphine, oxycodone, and fentanyl exhibit different analgesic profiles in mouse pain models. J. Pharmacol. Sci 111, 60–72. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A, 1985. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology 86, 274–280. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, Schindler CW, 2003. Reinstatement of punishment-suppressed opioid self-administration in rats: an alternative model of relapse to drug abuse. Psychopharmacology 168, 229–235. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, Schindler CW, 2005. Lorazepam reinstates punishment-suppressed remifentanil self-administration in rats. Psychopharmacology 179, 374–382. [DOI] [PubMed] [Google Scholar]
- Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, Giovino GA, West R, Hall W, Griffiths P, Ali R, Gowing L, Marsden J, Ferrari AJ, Grebely J, Farrell M, Degenhardt L, 2018. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 113, 1905–1926. [DOI] [PubMed] [Google Scholar]
- Peck JA, Galaj E, Eshak S, Newman KL, Ranaldi R, 2015. Environmental enrichment induces early heroin abstinence in an animal conflict model. Pharmacol. Biochem. Behav 138, 20–25. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Murray JE, Everitt BJ, 2013. Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. Eur. J. Neurosci 38, 3018–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelz WE, Whitney G, Smith JC, 1973. Genetic influences on saccharin preference of mice. Physiol. Behav 10, 263–265. [DOI] [PubMed] [Google Scholar]
- Phillips AG, McGovern DJ, Lee S, Ro K, Huynh DT, Elvig SK, Fegan KN, Root DH, 2019. Oral prescription opioid-seeking behavior in male and female mice. Addict. Biol e12828. [DOI] [PubMed] [Google Scholar]
- Radke AK, Held IT, Sneddon EA, Riddle CA, Quinn JJ, 2019. Additive influences of acute early life stress and sex on vulnerability for aversion-resistant alcohol drinking. Addict. Biol e12829. [DOI] [PubMed] [Google Scholar]
- Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE, McElligott ZA, McKlveen JM, Kash TL, Holmes A, 2017. Chronic EtOH effects on putative measures of compulsive behavior in mice. Addict. Biol 22, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Nakazawa K, Holmes A, 2015. Cortical GluN2B deletion attenuates punished suppression of food reward-seeking. Psychopharmacology 232, 3753–3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Adler MW, Gaughan JP, Baron A, Geller EB, Cowan A, 2003. NMDA receptors modulate morphine-induced hyperthermia. Brain Res 984, 76–83. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Benamar K, 2011. Effects of opioids, cannabinoids, and vanilloids on body temperature. Front. Biosci 3, 822–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savić Vujović KR, Vučković S, Srebro D, Ivanović M, Došen-Mićović L, Vučetić Č, Džoljić E, Prostran M, 2013. A comparison of the antinociceptive and temperature responses to morphine and fentanyl derivatives in rats. Arch. Pharm. Res 36, 501–508. [DOI] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G, 2018. Drug and opioid-involved overdose deaths - United States, 2013–2017. MMWR Morb. Mortal. Wkly. Rep 67, 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang S-J, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW, 2013. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat. Neurosci 16, 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Alvares K, Nespor SM, Grunberg NE, 1992. Effect of stress on oral morphine and fentanyl self-administration in rats. Pharmacol. Biochem. Behav 41, 615–619. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Klein LC, Alvares K, Grunberg NE, 1993. Effect of stress on oral fentanyl consumption in rats in an operant self-administration paradigm. Pharmacol. Biochem. Behav 46, 315–322. [DOI] [PubMed] [Google Scholar]
- Siciliano CA, Noamany H, Chang C-J, Brown AR, Chen X, Leible D, Lee JJ, Wang J, Vernon AN, Vander Weele CM, Kimchi EY, Heiman M, Tye KM, 2019. A cortical-brainstem circuit predicts and governs compulsive alcohol drinking. Science 366, 1008–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skupio U, Sikora M, Korostynski M, 2017. Behavioral and transcriptional patterns of protracted opioid self-administration in mice. Addiction. [DOI] [PubMed] [Google Scholar]
- Smith SG, Davis WM, 1974. Punishment of amphetamine and morphine self-administration Behavior. Psychol. Rec 24, 477–480. [Google Scholar]
- Sneddon EA, Ramsey OR, Thomas A, Radke AK, 2020. Increased responding for alcohol and resistance to aversion in female mice. Alcohol. Clin. Exp. Res [DOI] [PubMed] [Google Scholar]
- Sneddon EA, White RD, Radke AK, 2019. Sex differences in binge-like and aversion-resistant alcohol drinking in C57BL/6J mice. Alcohol. Clin. Exp. Res 43, 243–249. [DOI] [PubMed] [Google Scholar]
- Sustkova-Fiserova M, Puskina N, 2019. Ghrelin receptor antagonism of fentanyl-induced conditioned place preference, intravenous self-administration, and dopamine release in the nucleus accumbens in rats. Addiction Biol e12845. doi: 10.1111.adb.12845. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Crabbe JC, Boehm SL 2nd, 2014. “Drinking in the Dark” (DID): a simple mouse model of binge-like alcohol intake. Curr. Protoc. Neurosci 68, 9.49.1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton SR, Lohmann AB, Nicholson RA, Smith FL, 2000. Fentanyl self-administration in juvenile rats that were tolerant and dependent to fentanyl as infants. Pharmacol. Biochem. Behav 65, 563–570. [DOI] [PubMed] [Google Scholar]
- Varshneya NB, Walentiny DM, Moisa LT, Walker TD, Akinfiresoye LR, Beardsley PM, 2019. Opioid-like antinociceptive and locomotor effects of emerging fentanyl-related substances. Neuropharmacology 151, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veilleux JC, Colvin PJ, Anderson J, York C, Heinz AJ, 2010. A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin. Psychol. Rev 30, 155–166. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF, 2012. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J. Neurosci 32, 7563–7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CL, Schuster DJ, Domingo KM, Kitto KF, Fairbanks CA, 2008. Supraspinally-administered agmatine attenuates the development of oral fentanyl self-administration. Eur. J. Pharmacol 587, 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman AR, Arout C, Caldwell M, Dahan A, Kest B, 2009. Acute and chronic fentanyl administration causes hyperalgesia independently of opioid receptor activity in mice. Neurosci. Lett 462, 68–72. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, 1991. An ethopharmacological approach to the development of drug addiction. Neurosci. Biobehav. Rev 15, 515–519. [DOI] [PubMed] [Google Scholar]
- Xie Q, Buck LA, Bryant KG, Barker JM, 2019. Sex differences in ethanol reward seeking under conflict in mice. Alcohol. Clin. Exp. Res 43, 1556–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanni G, Featherstone BS, DeSalle MJ, Deutsch HM, 2019. Development and validation of a novel oral oxycodone self-administration protocol for female and male rats. bioRxiv. [Google Scholar]