Fig. 1. The allosteric cycle of the eukaryotic chaperonin CCT and conformational states visited during the cycle.
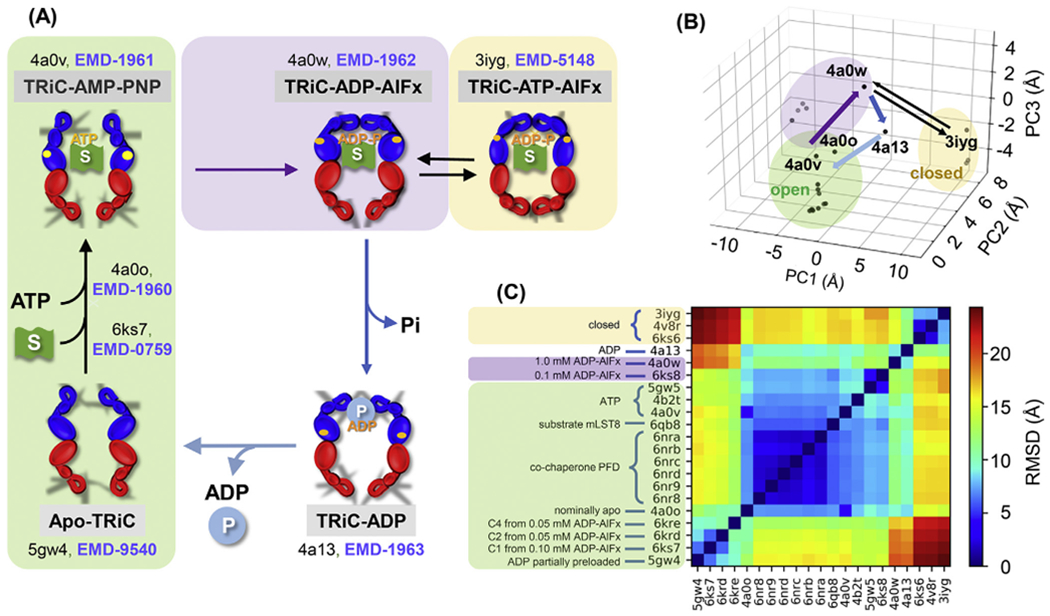
(A) Major conformational transitions triggered by ATP and substrate (S; green flag) binding and ATP hydrolysis (upper part). The upper left conformation represents the ATP-bound form as AMP-PNP (PDB: 4a0v) and ATP-γ-S (PDB: 4b2t) are non-hydrolysable ATP analogues. ATP hydrolysis is accompanied by ring closure (upper right); treatment with ADP-AlFx and ATP-AlFx (containing various aluminium fluorides) generates analogues corresponding to different stages in the hydrolysis process. Then phosphate release leads to ring opening again in the presence of the remaining ADP (lower right). This is followed by release of ADP and folded product (P; blue sphere), returning the heteromeric structure to the apo state (lower left). PDB and EMDB IDs are labelled for key resolved states. An ensemble of intermediate structures has been resolved for the ATP binding transition (including PDB: 6ks7) (Jin et al., 2019a). (B) Projection of all known structures onto the first three principal components (PC1-PC3) deduced from the principal component analysis (PCA) of the known conformers (each represented by a dot). The purple, dark blue and light blue arrows show the primary pathway between structures with at least one ring open, and the thin black equilibrium arrows display the passages between the kinetically trapped, fully closed conformations. (C) RMSDs between all pairs of structures shown in B. Green, yellow and purple shaded regions refer to conformers in open, closed and intermediate states in all panels.
