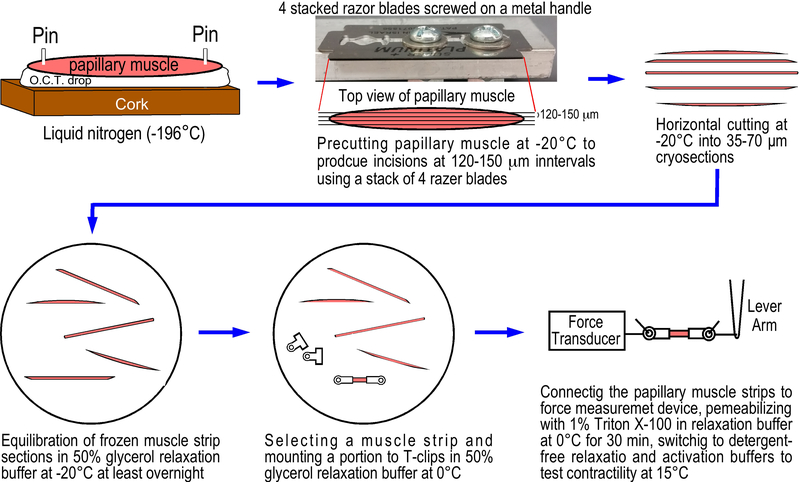
Figure.1. Preparation of skinned mouse left ventricular papillary muscle strips from cryosections.
A freshly isolated mouse papillary muscle was pinned on a piece of cork on a drop of O.C.T. compound. After flash freezing in liquid nitrogen, the frozen muscle tissue block was transferred to a cryostat set at −20°C, precut from the top manually using a stack of 4 razor blades screwed tightly on a metal handle at 120–150 μm intervals (based on the original thickness of the blades) to make partial incisions along the long axis of the papillary muscle in a depth about one-third (~150μm) of the muscle diameter. The precut portion of the papillary muscle was cryosectioned horizontally to produce 35 μm or 70 μm thick and 120–150 μm wide frozen muscle strips. The frozen muscle strips were immediately transferred into −20°C cold 50% glycerol relaxation buffer in a 35 mm cell culture dish to equilibrate at −20°C for at least 24 hours. The muscle strips were then mounted with aluminum T-clips at 0°C in 50% glycerol relaxation buffer. After attaching to force transducer and length lever arm, the muscle strip was skinned for 30 min in 1% Triton X-100 in relaxation buffer at 4–6°C. After switch to detergent-free solutions, the preparation is ready for contractility studies at 15°C.