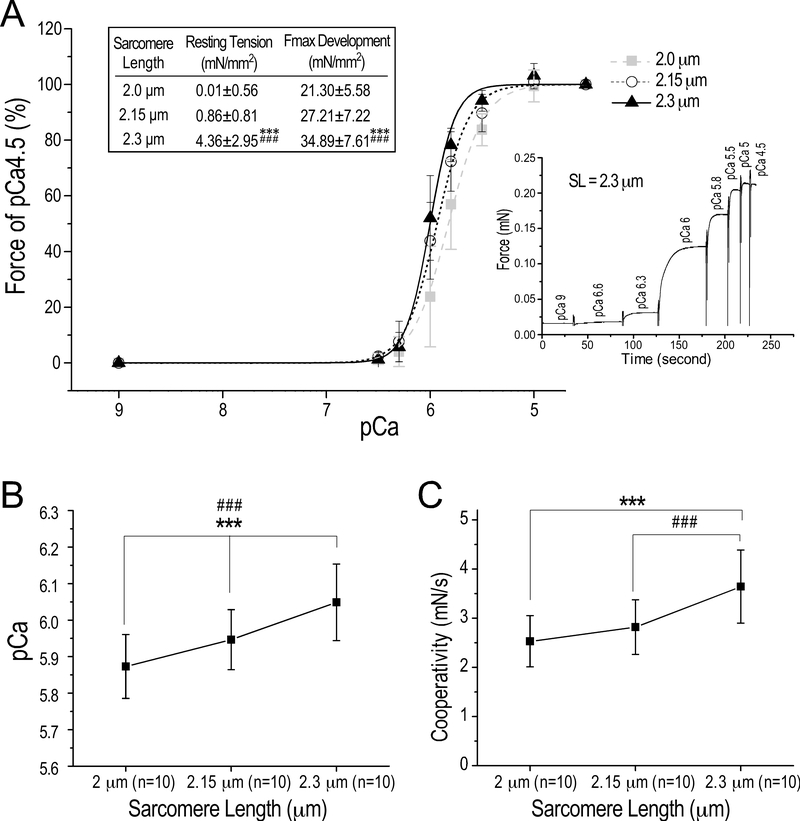
Figure 4. Preserved Ca2+-activated contractility of cryosection-generated skinned papillary muscle strips with positive responses to increase in sarcomere length.
(A) At SL of 2.0 μm, 2.15 μm and 2.3 μm, Ca2+ activated isometric contraction of skinned mouse papillary muscle strips was tested at pCa 9, 6.5, 6.3, 6, 5.8, 5.5, 5 and 4.5. A representative trace of the step increases of isometric force at increasing Ca2+ concentrations is shown. When SL was increased from 2.0 μm, to 2.15 μm and 2.3 μm, the cross-sectional area-normalized maximal tension development at pCa 4.5 of the muscle strips increased as expected for skinned fibers prepared from fresh cardiac muscle, indicating preserved functionality. The Hill fitted plots demonstrate corporative force development with left shifts of the force-pCa curve when SL was increased from 2.0 μm to 2.15 μm and to 2.3 μm, reflecting increases in Ca2+ sensitivity at longer SL (inset table). (B) pCa50, i.e., the Ca2+ concentration for 50% maximum force production in the Hill fitted force-pCa curve, increased significantly when SL was increased. (C) The cooperativity of Ca2+ activation of contraction also increased at longer SL, indicating increased myofilament contractility. The values are presented as mean± SD. N = 10 strips from 6 mouse hearts. ***P<0.001 vs SL 2.0 μm and ###P<0.001 vs SL 2.15 μm in one-way ANOVA repeated measures with pos hoc test.