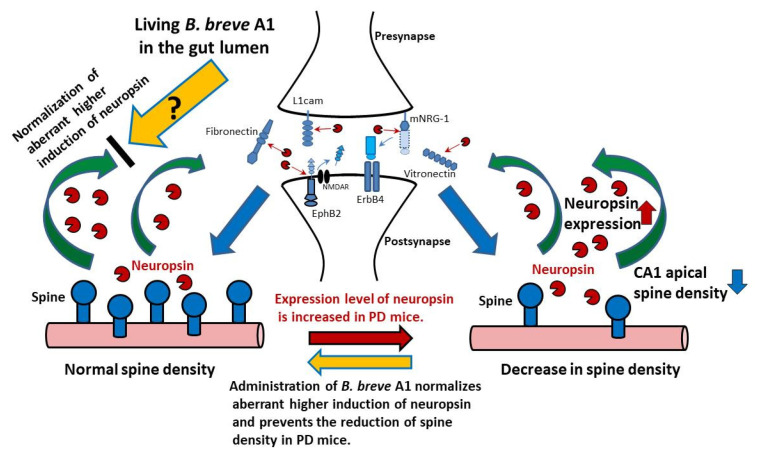
Figure 9.
Living B. breve A1 in the gut lumen leads to the restoration of PD-induced aberrant higher induction of neuropsin, the mechanism of which is caused by a still unknown signal via the gut–brain axis. Neuropsin-mediated signaling plays an important role in synaptic plasticity; physiologically activated neuropsin cleaves extracellular proteins in the synaptic cleft such as fibronectin, vitronectin, L1cam, EphB2 (the cleavage of EphB2 reduces EphB2-NMDA binding), and mNRG-1(the released moiety cleaved from mNRG-1 binds ErbB4 and triggers its phosphorylation), and modifies pre- and postsynaptic interactions and synaptic signal transmission [22,34]. However, an aberrant higher induction of neuropsin observed in PD mice causes abnormal changes in hippocampal synaptic plasticity and function. Oral administration of B. breve A1 restored the facilitation of contextual fear extinction and the decreased hippocampal dendritic spine density in PD mice via normalization of an aberrant higher induction of neuropsin. Abbreviations: EphB2, EPH receptor B2; ErbB4, NRG-1 receptor p185; L1cam, L1 cell adhesion molecule; mNRG-1, mature neuregulin-1; NMDAR, N-methyl-d-aspartic acid receptor.