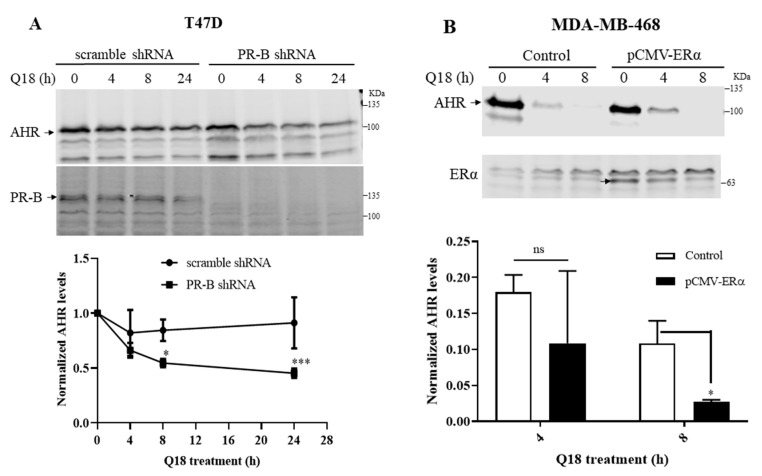
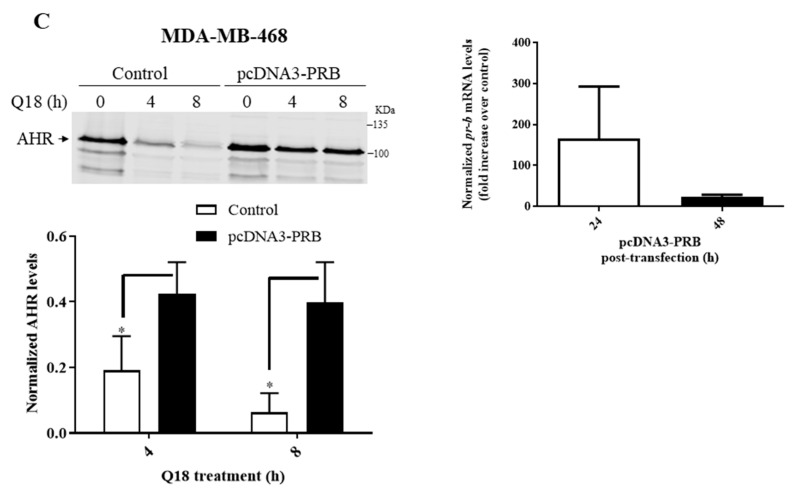
Figure 2.
Effect of PR-B and ERα on the Q18-mediated suppression of the AHR protein levels. Timecourse Q18 treatment of: (A) T47D stable cells carrying either PR-B-specific shRNA or scramble shRNA. AHR at zero time was arbitrarily set to one for comparison. (B) MDA-MB-468 cells transiently transfected with the plasmid carrying the full length ERα cDNA (pCMV-ERα). Control represents non-transfected cells. (C, left panel) MDA-MB-468 cells transiently transfected with the plasmid carrying the full length PR-B cDNA (pcDNA3-PR-B) or the pcDNA3 plasmid which was derived from pcDNA-PR-B after Srf I and Eco RV digest to remove 75% of PR-B cDNA (control). All lanes for Western blot analysis contain 60 μg of whole cell lysates. All Western blot (A–C) data of AHR were normalized by total protein stain. All the results are means ± SD of three independent experiments. Two-way ANOVA with Sidak’s multiple comparisons test was used in 2A for statistical analysis. * p < 0.05, *** p < 0.001 when compared between PR-B shRNA and scramble shRNA. Unpaired t test was used in 2B and 2C for statistical analysis. ns, not significant. * p < 0.05 when compared to control. The pr-b message levels were determined by RT-qPCR 24 or 48 h post-transfection, normalized by 18S (C, right panel). Cq of pr-b message 24 h after post-transfection were 32–33 whereas 48 h after post-transfection were 35–36. The Cq of pr-b message in untransfected cells were all above 39. Y-axis represents a fold increase in pr-b messages of PR-B transfected over empty plasmid transfected. Although the PR-B-transfected cells consistent showed more pr-b message, all Cq numbers were beyond 30, showing that the amount of all pr-b messages were all very low so that no statistics was performed.