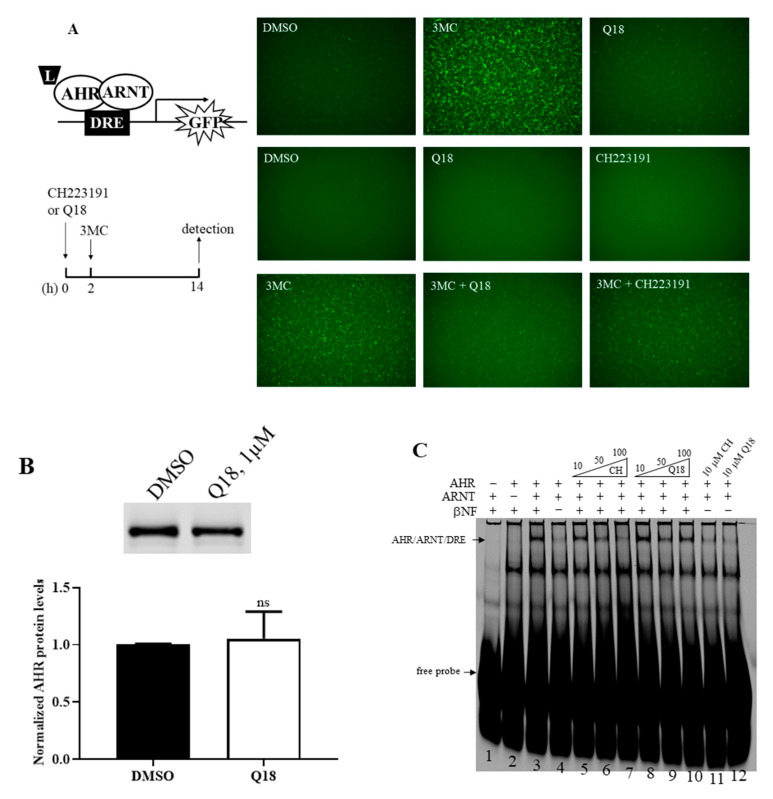
Figure 3.
Effect of Q18 on AHR ligand binding. (A) Treatment of Q18, CH223191, and 3MC in rat H4G1.1c3 cells stably transfected with a plasmid carrying a DRE-driven GFP cDNA (left top diagram). Cells were treated for 12–14 h (as indicated in the left bottom timeline diagram) with DMSO, 1 μM 3MC, 1 μM Q18, 10 μM CH223191, 1 μM 3MC plus 1 μM Q18, or 1 μM 3MC plus 10 μM CH223191. Q18 could not cause GFP formation but could block the GFP formation caused by 3MC. CH223191 was used as the AHR antagonist control. Top panel represents one experiment; middle and bottom panels represent another experiment. These two experiments were repeated two more times with similar results. (B) Western blot analysis to show the effect of Q18 on the AHR protein levels when the stable H4G1.1c3 cells were treated with 1 μM of Q18 for 24 h. The AHR protein levels were normalized by total protein stain. The error bar represents the mean ± SD of three independent experiments and the representative Western images are shown. (C) Effect of Q18 on the formation of the AHR/ARNT/DRE gel shift complex. Gel shift complex was formed in the presence of 10 μM of βNF as the AHR ligand (lanes 1–4). A total of 10 μM of Q18 could not form the AHR gel shift complex but could suppress the βNF-dependent AHR gel shift formation at a 50 μM concentration (lanes 8–10 and 12). CH223191 (CH) was used as the AHR antagonist control (lanes 5–7 and 11). Arrows indicate the AHR/ARNT/DRE gel shift complex and free DRE conjugated with IRD700 (free probe). This gel shift experiment was repeated once with similar results. ns, not significant.