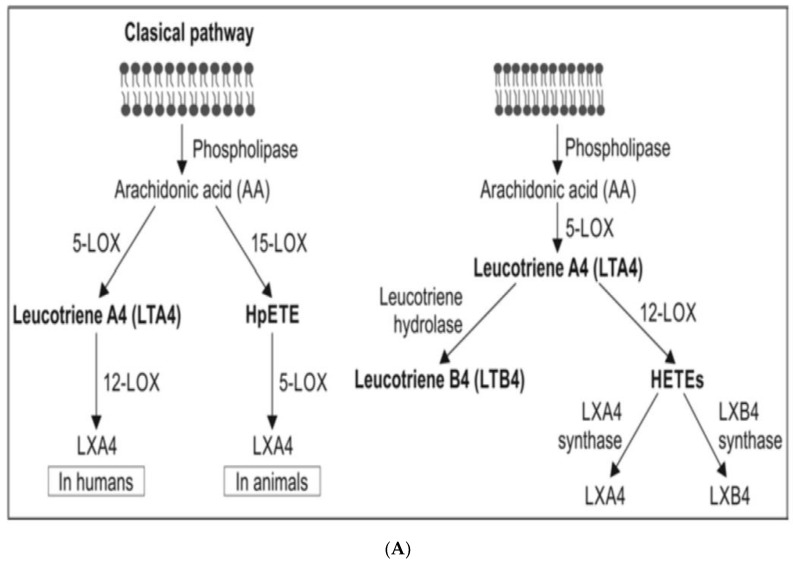
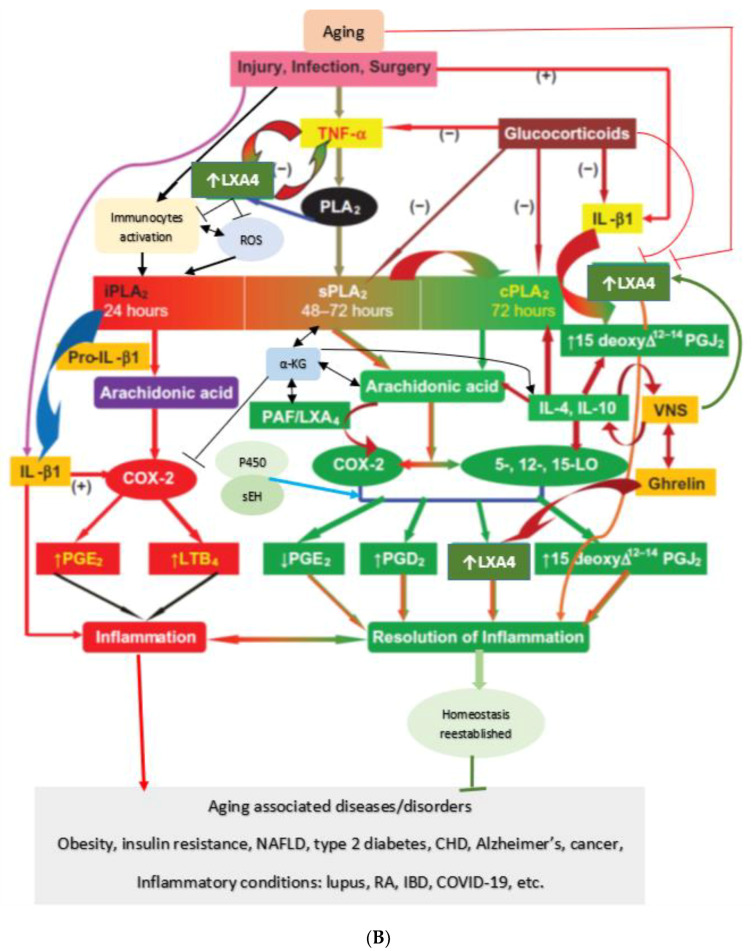
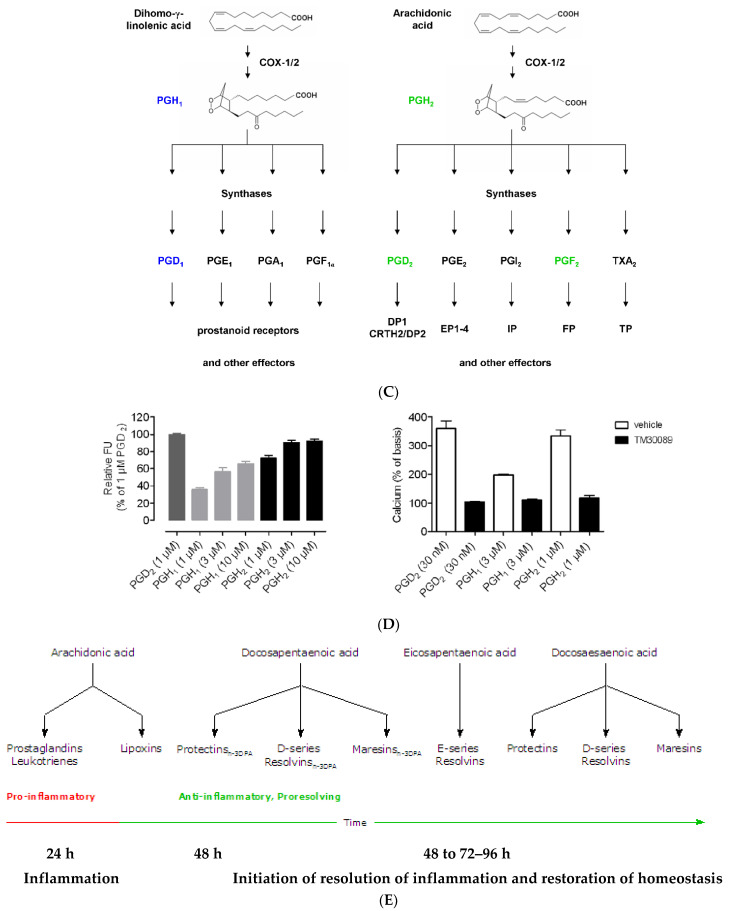
Figure 3.
(A) Scheme showing the metabolism of AA and how pro-inflammatory LTA4 can be converted to anti-inflammatory LXA4. Similar conversion of pro-inflammatory PGs, TXs and LTs to anti-inflammatory resolvins, protectins and maresins may occur. (B) Scheme showing interaction(s) among BALs, cytokines, glucocorticoids, and inflammatory process. (-) Indicates inhibition of action or synthesis; (+) indicates increase in synthesis or action. sEH = soluble epoxide hydrolase; VNS = vagal nerve stimulation; ROS = reactive oxygen species. α-KG = alpha-ketoglutarate. Infections, surgery, and injury activate PLA2 leading to the release of AA and other unsaturated fatty acids from the cell membrane lipid pool. Simultaneously circulating leukocytes, macrophages and other immunocytes are activated that release IL-6 and TNF-α, which stimulate PLA2 leading to release of AA (and DGLA, EPA, DPA an DHA). COX-2 and LOX enzymes convert DGLA, AA, EPA and DHA to form their respective metabolites. To balance the action of pro-inflammatory cytokines, there will be release of anti-inflammatory cytokines IL-4 and IL-10. Pro-IL-β1 released is converted to IL-β1, a pro-inflammatory cytokine. IL-β1 acts on cPLA2/sPLA2 to induce the release of second wave of AA from the cell membrane that can be converted to LXA4, an anti-inflammatory molecule. During the first 24 h of injury, infection, and surgery, iPLA2 is activated. AA and other PUFAs released due to the activation of iPLA2 are utilized mainly to form pro-inflammatory bioactive lipids. In contrast to this, 48–96 h after infection, injury, and surgery activation of cPLA2/sPLA2 occurs that induce the release of second wave of AA and other PUFAs (polyunsaturated fatty acids) that is used to form anti-inflammatory LXA4, PGD2 and PGJ2. This switchover from pro-inflammatory PGE2 and LTs to anti-inflammatory LXA4 is facilitated by PGE2. Once the local tissue and plasma concentrations of PGE2 reach the peak, it (PGE2) activates 5-LOX and 15-LOX enzymes to enhance the formation of LXA4 to initiate resolution of inflammation and enhance wound healing. If PGE2 concentrations fail to reach its peak levels, stimulation of 5- and 15-LOX fails to occur, and so inflammation persists. It is likely that once the PGE2 levels reach their peak, it stimulates cPLA2 and sPLA2 enzymes to induce second wave of AA release. EPA, DHA and DGLA may have actions like AA (not shown in the figure). EPA forms the precursor to 3 series PGs, TXs and 5 series LTs (that are less pro-inflammatory compared to 2 series PGs, TXs and 4 series LTs formed from AA but are nevertheless pro-inflammatory) and resolvins of E series that are anti-inflammatory. DHA forms precursor to resolvins, protectins and maresins all of which are potent anti-inflammatory molecules. DGLA is the precursor of PGE1, an anti-inflammatory molecule, that has actions like LXA4 (see Table 1 and Figure 3C). This figure is modified from reference [37]. (C) Scheme showing the metabolism of DGLA and their metabolites. PGH1 has pro-inflammatory actions but is less potent compared to PGH2 and PGE2. PGE1 is anti-inflammatory in nature. (D) PGH1 stimulates Ca2+ mobilization in CRTH2 transfected and primary eosinophils. It is evident that PGH1 is less potent compared to PGH2 and so is less pro-inflammatory compared to PGH2 and PGE2. This data is taken from PLoS ONE 2012, 7, e33329, doi:10.1371/journal.pone.0033329. (E) Summary of metabolites formed from AA, DPA, EPA and DHA and possible timeline of their formation during inflammation and resolution phases of inflammation. It may be noted that once the resolution process of inflammation starts, there is a need for removal of debris, regeneration of tissues (stem cells need to move to the site of inflammation, proliferate, differentiate and induce repair of damaged tissues and restore homeostasis). It is likely that lipoxins are needed for inhibition of inflammation; resolvins for resolution of inflammation; protectins for protecting normal tissues; and maresins for stem cell function. All these events may occur simultaneously but in a highly coordinated and regulated fashion. It is proposed that the concentrations of various molecules involved in inflammation and its resolution and restoration of homeostasis is as follows: 24 h: PGE2↑↑↑↑; LXA4↑; RSVs; PRTs; MaRs. 48 h: PGE2↑↑↑; LXA4↑↑; RSVs↑; PRTs↑; MaRs↑. 72 h: PGE2↑↑; LXA4↑↑↑; RSVs↑↑; PRTs↑↑↑; MaRs↑↑↑. 96 h: PGE2↑; LXA4↑↑; RSVs↑↑↑; PRTs↑↑↑; MaRs↑↑↑↑. >96 h: PGE2↑; LXA4↑; RSVs↑↑; PRTs↑↑; MaRs↑↑↑. The actions of these compounds in the inflammation and wound healing process can be as follows: LXA4 → anti-inflammatory >resolution >protection >proliferation. RSVs → resolution > anti-inflammatory >protection >proliferation. PRTs → protection > resolution > anti-inflammatory > proliferation. MaRs → proliferation > protection > resolution > anti-inflammatory. Resolution refers to resolution of inflammation. Protection refers to protection of normal cells/tissues from injurious agents. Proliferation refers to proliferation of stem cells and other cells to replace damaged cells/tissues. Even though all compounds have similar and overlapping actions and possess anti-inflammatory properties, each lipid may show one particular action more compared to the other actions.