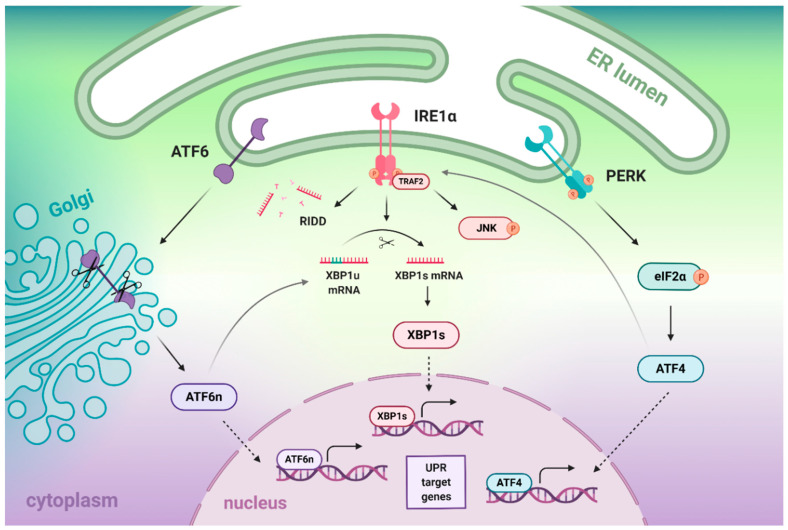
Figure 1.
The structure of the Unfolded Protein Response (UPR)-dependent signaling branches and the crosstalk between them. Upon ER stress, the activating transcription factor 6 (ATF6) undergoes proteolytic cleavage in the Golgi apparatus, wherein its transcriptionally active form (ATF6n) is produced. The inositol-requiring enzyme type 1α (IRE1α) possesses several activities: It induces the regulated IRE1-Dependent Decay (RIDD) of specific mRNAs, the unconventional splicing of x-box binding protein 1 (XBP1) mRNA, as well as it interacts with the TNF Receptor Associated Factor 2 (TRAF2) to initiate the c-Jun N-terminal kinase (JNK) signaling cascade. Activated protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) phosphorylates the eukaryotic initiation factor 2α (eIF2α), which blocks general translation initiation and selectively induces translation of particular mRNAs at the same time, including activating transcription factor 4 (ATF4). The downstream targets of all UPR branches, namely ATF6n, ATF4, and the spliced form of XBP1 (XBP1s), translocate to the nucleus to regulate the transcription of UPR target genes. Additionally, both ATF6n and ATF4 upregulate IRE1α activity, via induction of XBP1 mRNA and increase in IRE1α expression, respectively.