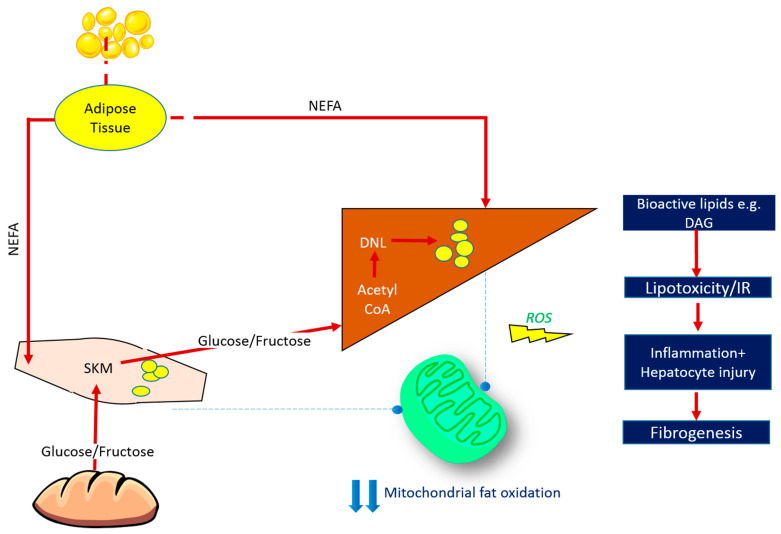
Figure 1.
Fate of Ingested Macronutrients in States of Chronic Energy Surplus. Insulin resistance arises when nutrient storage pathways, which evolved to maximise efficient energy utilisation, are exposed to chronic energy surplus. Ectopic lipid accumulation in liver and skeletal muscle disrupts insulin signalling in these tissues, leading to reduced muscle glucose uptake and impaired hepatic glycogen synthesis. Muscle insulin resistance diverts ingested glucose to the liver, resulting in increased hepatic de novo lipogenesis and hyperlipidaemia. Increased liver fat stores are thus attributable to both increased free fatty acid (FFA) flux from insulin-resistant adipose tissue, and also diversion of dietary carbohydrate away from muscle glycogen synthesis and towards lipogenic pathways in the liver. The most biologically active form of liver fat is diacylglycerol, which is probably the moiety most directly responsible for impaired insulin signalling, with triglyceride being relatively metabolically inert. Reduced mitochondrial oxidative capacity in these tissues diminishes the ability of stored lipids to undergo oxidation, further exacerbating lipotoxicity and contributing toward oxidative stress. This culminates in necroinflammation and the onset of hepatic fibrogenesis. ROS = reactive oxygen species, SKM = skeletal muscle, NEFA = non-esterified fatty acids, DNL = de novo lipogenesis, DAG = diacylglycerol, IR = insulin resistance.