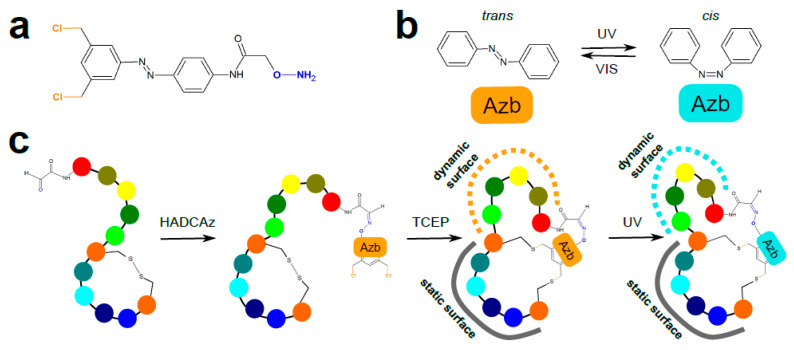
Figure 6.
The use of azobenzene-based crosslinker to construct light-responsive bicyclic peptides (adapted from [46]). (a) A tridentate hydroxyl amine/dichlorobenzene-containing azobenzene (HADCAz) crosslinker. (b). Ultraviolet (UV) light-induced reversible geometric (configurational) isomerization of azobenzene (Azb). (c) Sequential bicyclization of N-terminally glyoxal-functionalized peptide with the HADCAz crosslinker. TCEP—reducing agent tris(2-carboxyethyl)phosphine used to break the disulfide bond.