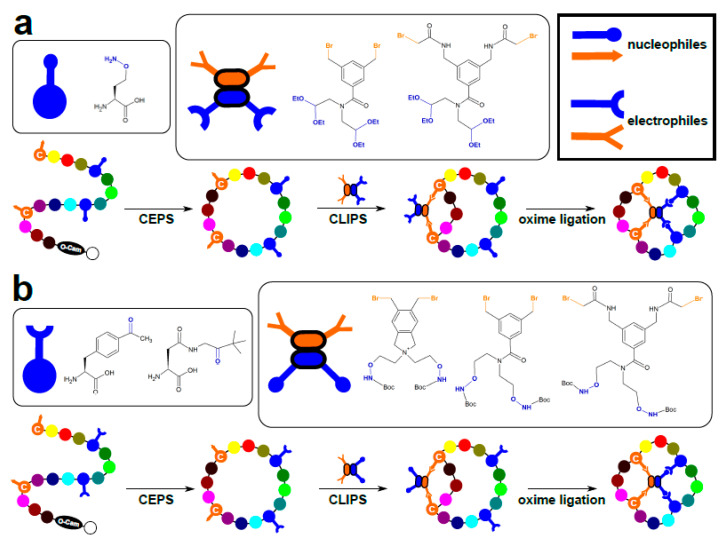
Figure 7.
Three-stage macrocyclization of unprotected peptides, combining chemo-enzymatic peptide synthesis using omniligase-1 (CEPS), chemical ligation of peptides onto scaffolds (CLIPS), and oxime ligation (adapted from [58]). Two distinct strategies are depicted, differing in CLIPS crosslinkers and cognate residues harboring orthogonally reactive functional groups. (a). CLIPS with dibromo-/dicarbonyl crosslinkers, and aminooxy-homoserine residues. (b). CLIPS with dibromo-/di(aminooxy) crosslinkers and carbonyl group-containing residues. C—cysteine, O-Cam—carboxamidomethyl (Cam)-ester, Boc—tert-butyloxycarbonyl.