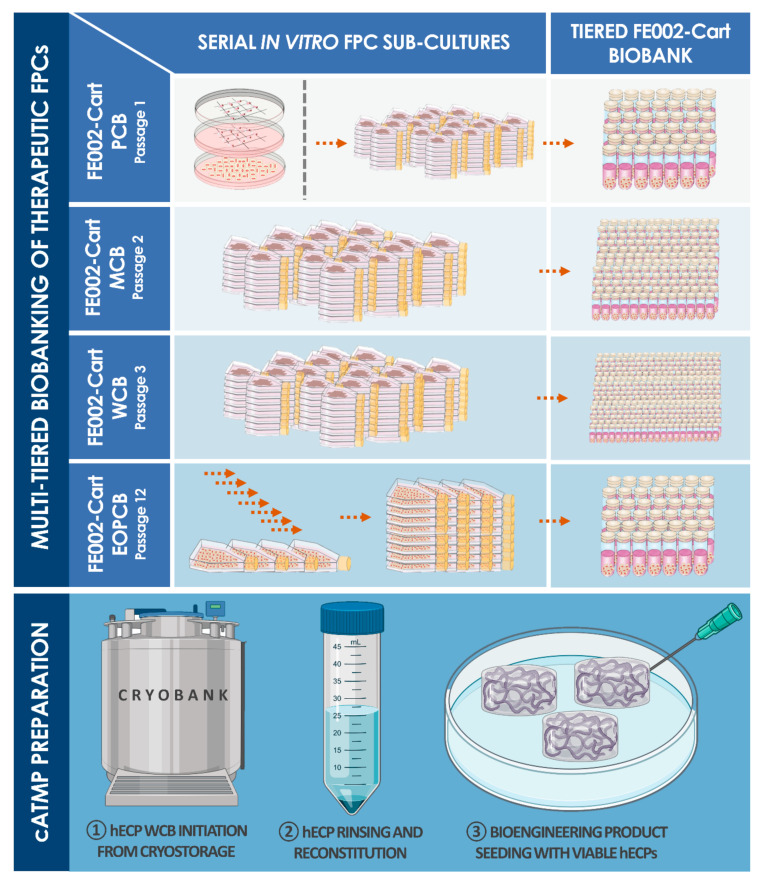
Figure 2.
Schematic workflow for optimized and standardized multi-tiered cell banking of primary hECPs (e.g., non-enzymatically isolated FE002-Cart cell type) and cATMP preparation for the clinic. In vitro optimization steps in the pilot expansion study allow one to select the optimally adapted serum manufacturer and lot, two-dimension culture surface size and brand, producing the overall best efficiency of manufacturing. Defined technical specifications and rigorous screening, characterization testing, batch release testing, and safety testing allow for the liberation of highly homogenous, stable, and safe therapeutic biological materials for use in regenerative medicine applications. These considerations and technical specificities are largely dependent on the inherent high robustness, consistency, and stability of properly harnessed FPC sources. Industrial transposition to GMP manufacturing is tangibly attained with such materials, whereas extensive multi-tiered cryopreserved cell banks may be rapidly and efficiently established as proposed herein. Presented bank tiers and passage nomenclature were validated for the FE002-Cart cell type. Off-the-freezer use of such FPC sources enables simple, extemporaneous, and the on-demand production of cATMPs.