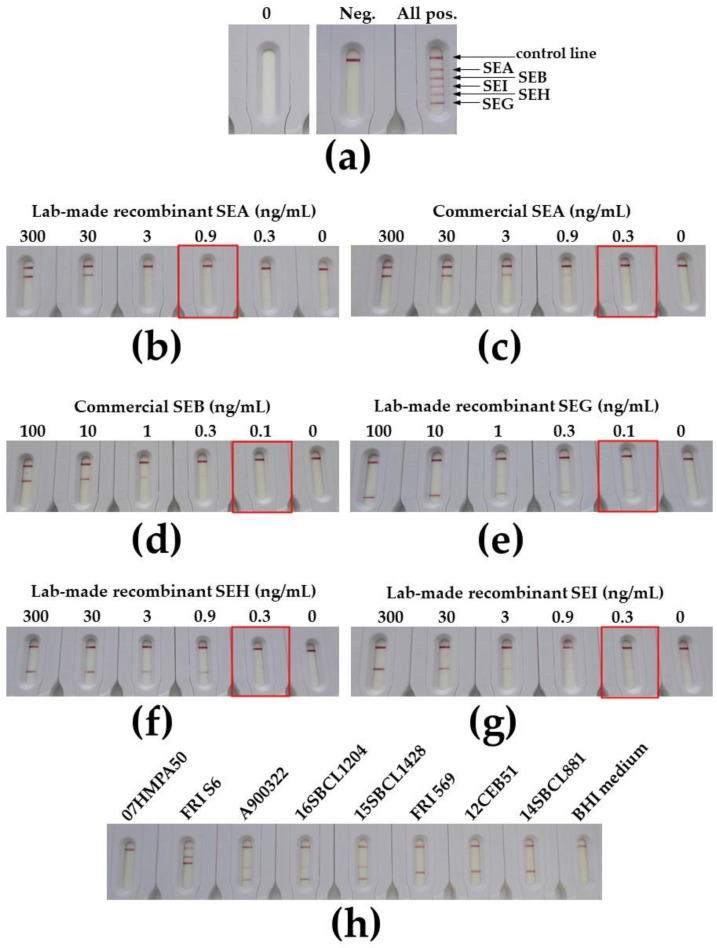
Figure 6.
Detection of SEA, SEB, SEG, SEH, and SEI using multiplex immunochromatographic test after 30 min of migration. (a) Description of the manufactured multiplex cassette: 0, cassette before use; Neg., negative result; All pos., positive result for all enterotoxins (solution containing 3 ng/mL for each SEA, SEH, and SEI and 1 ng/mL for each SEB and SEG in ICT buffer). Serial dilutions with lab-made recombinant SEA in (b), commercial SEA (c), commercial SEB (d), lab-made recombinant SEG (e), SEH (f), or SEI (g) toxin were prepared in immunochromatographic (ICT) buffer before loading 100 μL onto the cassette. (h) BHI-cultured S. aureus supernatants were processed as described in the methods section before performing the test by loading 100 μL onto the cassette. The monoclonal antibody (mAb) pairs used in these manufactured (NG-Biotech, France) multiplex cassettes were the same as for the lab-made monoplex strips.