FIG 3.
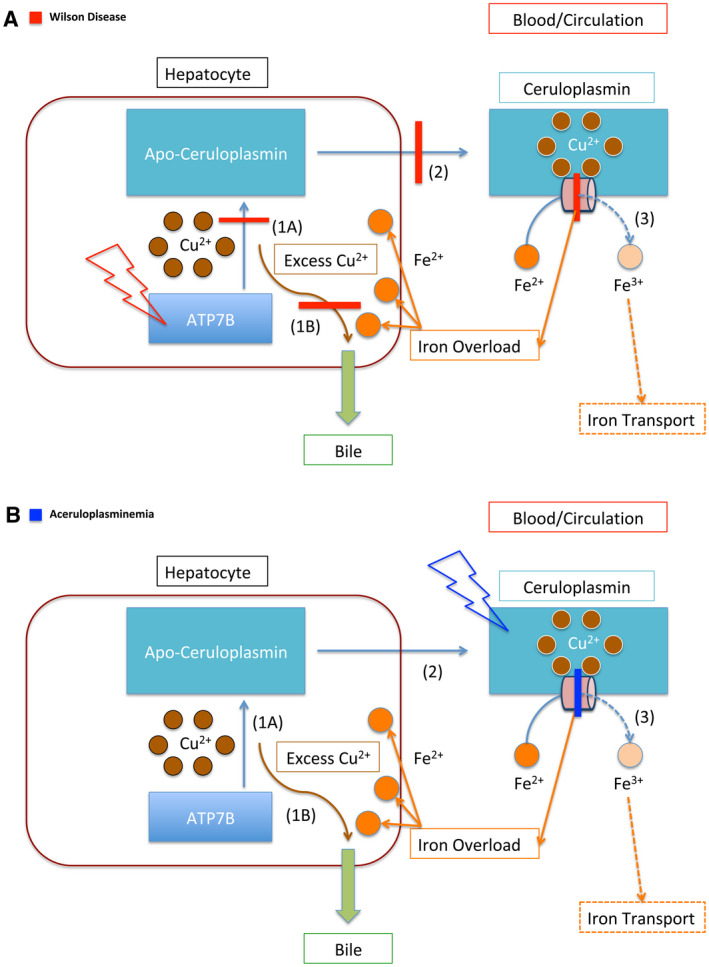
Copper and iron transportation in WD and ACP. (A) Schematic representation of the impaired copper and iron transportation in WD. The red lightning bolt represents mutation. This panel illustrates the mutation of the ATP7B protein, the primary defect in WD. The red bars represent the downstream effects of this protein mutation. In WD, ATP7B is unable to load copper onto Apo‐ceruloplasmin (1A), which prevents copper from being transported throughout the body (2). In addition, ATP7B is unable to facilitate the secretion of copper through the bile, which further causes copper accumulation within the hepatocyte (1B). Furthermore, because Ceruloplasmin lacks copper, it cannot ferroxidize iron (3). The impairment of ferroxidase activity is represented by the red bar across the peach cylinder. The dashed lines represent downstream effects of impaired ferroxidation, leading to iron accumulation in the liver. (B) Schematic representation of the impaired iron transportation in ACP. The blue lightning bolt represents the mutation of the protein Ceruloplasmin. The blue bar represents the impairment of Ceruloplasmin’s ferroxidase activity. As in (A), the dashed lines represent the impaired downstream effects of this, which is the inability of Ceruloplasmin to oxidize iron, which leads to impaired iron transport (3). In contrast with WD (A), the function of the ATP7B protein is not impaired, and it is able to transport copper (1A, 2). It retains the function of facilitating copper excretion through bile (1B). Thus, copper accumulation is not typically associated with ACP, whereas iron accumulation is.
