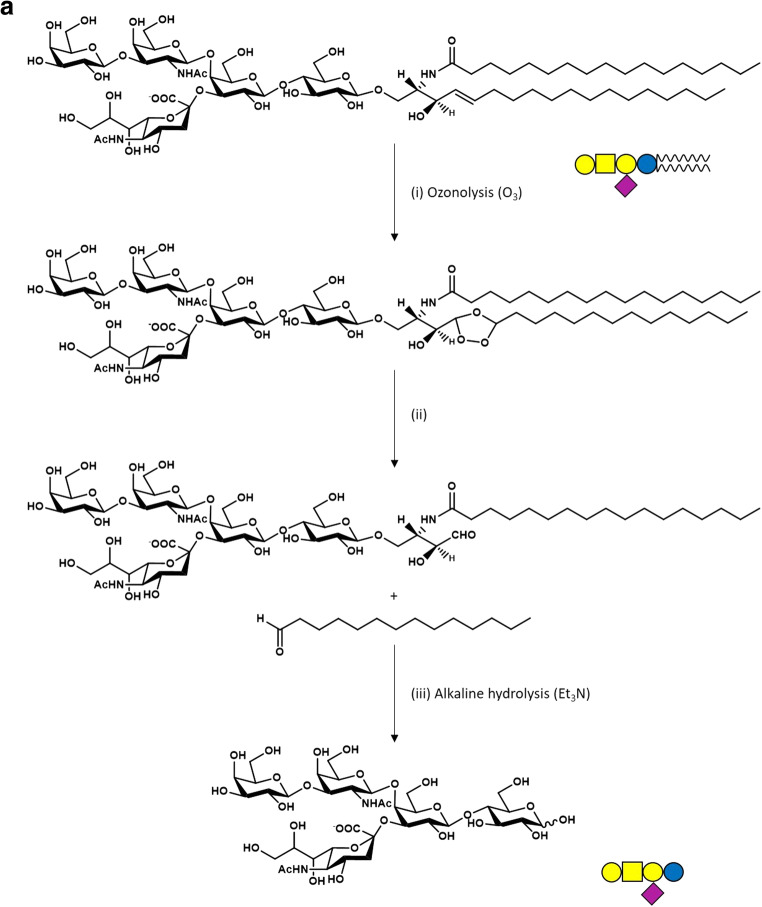
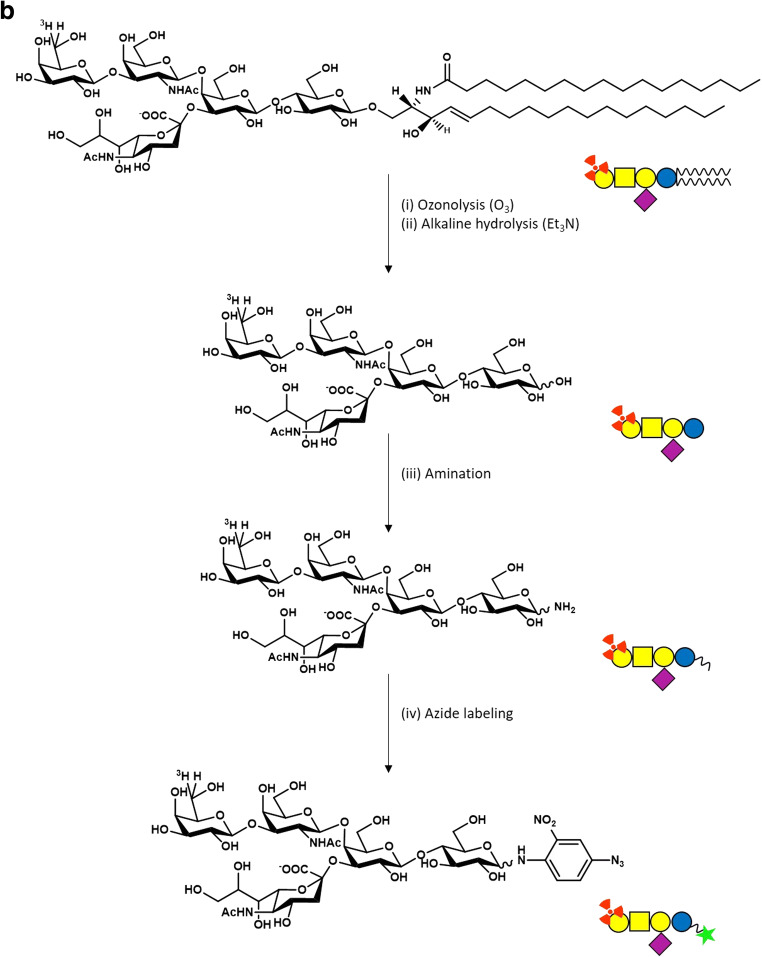
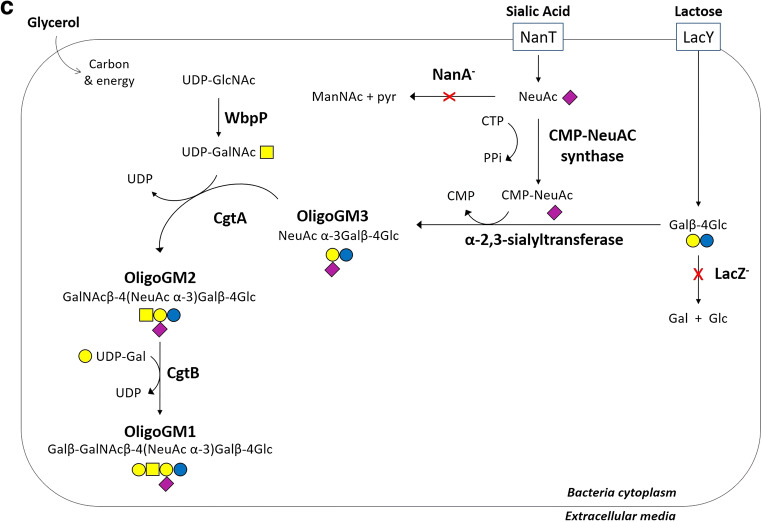
Fig. 2.
OligoGM1 synthesis strategies. a Scheme of the chemical synthesis of OligoGM1. GM1 undergoes to ozonolytic (O3) process in methanol (i) generating a desphingosine aldehyde product and a miristic aldehyde (ii), followed by alkaline hydrolysis in triethylamine (Et3N) (iii) releasing OlgoGM1; b Scheme of the chemical synthesis of tritium-labeled and photoactivable OligoGM1. [3H]GM1 undergoes ozonolysis (i) in methanol followed by triethylamine alkaline hydrolysis (ii) releasing [3H]OligoGM1. The latter is subjected to the amination process (iii) and the [3H]OligoGM1-NH2 is azide labeled (iv) with 2-nitro-fluorophenylazide in dimethylformamide (DMF) and tributiylamine (Bu3N) in dimethylsulfoxide (DMSO) to obtain [3H]OligoGM1-N3. c OligoGM1 biosynthesis in engineered Escherichia coli. Lactose and sialic acid (Neu5Ac) are internalized by the specific permeases LacY and NanT but cannot be degraded because of β-galactosidase (LacZ) and aldolase (NanA) deletion. CMP-Neu5Ac synthase activates Neu5Ac into CMP-Neu5Ac which is transferred onto lactose by α2,3-sialyltransferase (encoded by Lst), to form II3αNeu5Ac-Lac (sialyllactose, OligoGM3). The use of the endogenous pool of UDP-GalNAc produced by the recombinant UDP-GlcNAc C4 epimerase (WbpP) allows β1,4-GalNAc transferase (CgtA) to catalyze the glycosylation of sialyllactose to form II3αNeu5Ac-Gg3 (OligoGM2). This compound is substrate for the β1,3-galactosyltransferase (CgtB) to yield II3αNeu5Ac-Gg4 (OligoGM1). CTP, cytidine triphosphate; Ppi, inorganic pyrophosphate. Oligosaccharide sugar code is according to Varki et al. [36]