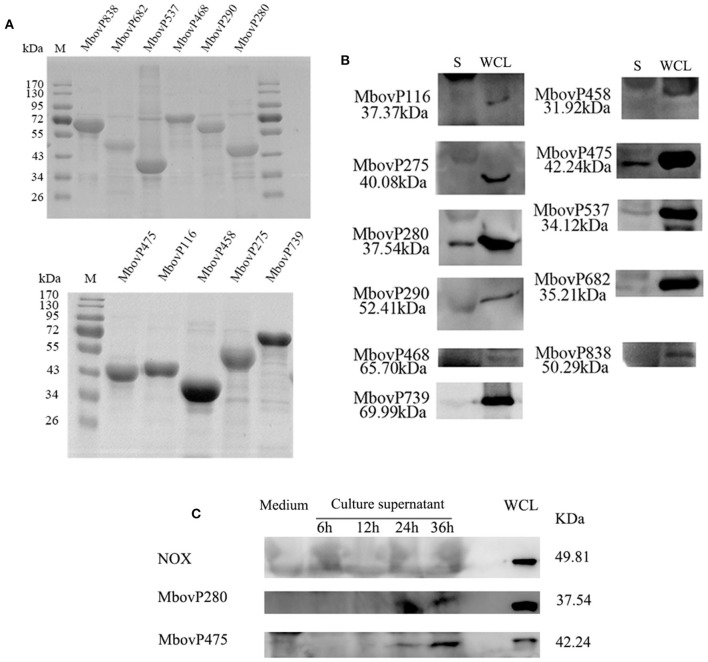
Figure 1.
Secretion of MbovP280. (A) Purification of 11 predicted secreted proteins. The proteins were purified with nickel affinity chromatography and resolved with SDS-PAGE. (B) Confirmation of the predicted secreted proteins with western blotting assays. Secretome (S) and whole-cell lysate (WCL) of M. bovis were resolved with SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Polyclonal antibodies directed against rMbovP280, rMbovP290, rMbovP475, rMbovP468, rMbovP838, rMbovP537, rMbovP458, rMbovP682, rMbovP116, rMbovP275, and rMbovP739 were used to detect the proteins in the secretome. (C) Visualization of the secreted MbvoP280 in culture supernatant. M. bovis HB0801 was cultured in PPLO medium. The culture supernatant was collected and concentrated at 6, 12, 24, and 36 h after incubation. The antiserum against rMbovP280 and rMbovP475 were used to detect the proteins in the supernatant, while M. bovis NOX known as the membrane-associated protein served as the negative control (NC).